
| Library |  |
|
|
Introduction. The
Dawn of Medicine
Survival, for most species, depends upon keen senses and a sharp eye. Our early ancestors� powers of observation would have been cultivated to a far higher level than is usual in modern societies. Very little would have escaped their attention. In that respect,
it may be conjectured that the animals were our first teachers. In the
mythology of all cultures, there is a good leavening of mythical beasts,
both sacred and symbolic. This totemism is clearly in evidence in all of the
earlier civilisations. In particular those of the Egyptian, Greek and
Assyrian and Babylonian. To this we must also add those lost civilisations
of the Far East and the Americas. It is commonplace to see the domestic cat and ingesting various types of grass in order to induce the vomiting of hair balls. Those sharp human eyes would have noted, that other species, when ailing, would have ingested various types of plants. In that context, it is interesting to note that the goat in European pagan mythology is known as the herbalist of the animal world. For a large proportion of herd animals, the changes of the seasons would bring with it a reoccurring pattern of migration. Perhaps the declination of the Sun produced a subtle change of light that released hormonal messengers to signal that it was time to make the long trek, upon which survival depended. The herd animals are excellent cartographers who will unerringly find their way over or around obstacles, whilst on the way from one watering hole to the next. Untold thousands of sharp hooves would have cut and tamped man kinds earliest trails and tracks. For those early people who tracked the herds, the changing vegetation would be noted. and added to the store of survival knowledge. Tenuous as it is, Shanidar, is the earliest known link to a proto-system of medicine. The pollen samples recovered represented 8 genera of flowering plants. This has led some writers to speculate that the motive for the flowering plants was pathos. I would like to suggest that the motive was pragmatism. For the following reasons; 1. Stone and bone tools were also recovered from the site. Those tools took considerable labour and skill to produce. Therefore, as barter goods they would have high value and would not be abandoned, unless there was some compelling reason. That reason may have been a journey of the soul to another region. The tools were survival artefacts for the soul on its journey to another world. 2. The pollen samples of the 8 different genera, were representative of medicinal herbs, which are still in use today. Therefore, the 2 main ingredients for survival on a long journey to the other side, were left at the burial site. Tools as survival artefacts and Plants as medicine. The remnants of Neolithic cultures and their practices exist today. Medicinal herbs are prepared by beating the fresh plant between 2 stones, either as a single plant, or as a compound of plants. The herbs are reduced to a paste. The paste may be applied externally as a poultice. Alternatively, crude pills may be formed from the paste, however, the ingestion of plants was the exception, rather than the rule, in those Neolithic cultures. The reason was very simple, the dermal route of administration was the safest. Early Celtic herbalists used Foxglove ointment to cure dropsy. The problems of posology were minimised and a potentially lethal herb was tamed. The early roots of compounding are to be found in the dust of the Neolithic peoples. The founding date of the Sumerian City States, scattered along the banks of the Euphrates and the Tigris, is not known. However, a carved stone frieze, which depicts the hand pollination of date palms, has been dated, between 8 and 9000 years old. This indicates a sophisticated knowledge of plant reproduction techniques. Wherever people gather together in large numbers, problems of logistics and supply will arise. Not the least of these, would be the provision of medical care and medicines. We may be certain that the compounding of medicinal plants had advanced far beyond the Neolithic practices. The earliest written records have been dated at 5000 years. The records were in the form of baked clay tablets. Some of the tablets were pharmaceutical recipes. The construction of those recipes display a fine grasp of the art of compounding. It should be said, that we have no firm knowledge of the early development of those City States. Compounding may lay claim to be the foremost of the pharmaceutical arts, and is the foundation upon which pharmacy, both ancient and modern, rests. Compounding is the combining of two or more ingredients, in a suitable vehicle, for administration to a patient. This seemingly simple operation, would in fact, need a good leavening of skill and knowledge. Overcoming incompatibilities in ingredients, and the vehicle used to administer a remedy, is the crux of the matter.
|
The Standardised Remedy 11.6
The active ingredient of the
remedy must be standardised so that the correct dose can be administered. The
method to be used is arbitrary
standardisation, the method is covered in
Module 8.5. In this way, the synergy
of the extract remains intact.
Posology or Dose 11.7
Here a word of warning :
If one should one prepare or dispense any herb that is included in a
Restricted or Poisons List, except as a Homoeopathic preparation.
There could be considerable legal fallout. Examples of such plants have been
covered in a previous module.
It is of utmost importance that one is thoroughly conversant with the Pharmaceutical calculations that are covered in Paragraphs 7.43 through 7.60. The dosage is the responsibility of the Prescriber, and not that of the Dispenser. However, the dispenser should satisfy themselves that the dosage is in the recognised range. Therefore, a brief discussion of the subject is in order.
Orthodoxy ignores synergy, and proceeds from the standpoint of �Active Principle�. In short, the efficacy of a plant drug is measured by its content of a single constituent, usually an Alkaloid or Glycoside. The bulk of the worlds� supply of a particular plant drug is grown in specific regions around the globe. Consequently, the level of the active ingredient within a particular specie of herb is a function of known variables, and from that, a plus or minus percentage for those levels are known.
That variation is used by the Science of Posology to produce a chemically standardised medicine. LD 50 tests are then used to arrive at a safe dose for an average adult. Dosages may be gleaned from the older Dispensatories, Extra Pharmacopoeias or Pharmaceutical Codex, for whatever nation. Recommended as a handy bench top reference, is a British publication which is updated regularly :
Potters New Cyclopedia of Botanical Drugs and Preparations.
C. W. Daniel
Company Limited
Saffron Walden, Essex
England.
When a safe, functional adult dose has been established, then there is the matter of dosage for children. There are various methods used to arrive at a proportional dose for a child, e.g.,
1.
Clark�s Rule:
Used for children of 2 years
down to 1 of age, and is based upon body weight.
Adult dose x Weight of Child
150
2.
Young�s Rule :
For children over 2 years of
age, and is based on age in years.
Adult dose x Child�s age.
Child�s age + 12
| Age | Proportional Dose |
| Adult | 1 = 100% |
| 12 Years | � = 50% |
| 8 Years | 2/5 = 40% |
| 4 Years | 1/4 = 25% |
| 2 Years | 1/8 = 12.5 % |
| 1 Year | 1/16 = 6.25% |
Breast fed infants under 1 year, would normally take the medicine through the mothers milk. The mother would take the adult dose and the child would receive a proportional dose across a 24 hour period..
4. The
Surface Area Rule :
The height and weight of the child is measured and
then the figures are referred to a surface area chart. When the surface
area is determined it is applied as follows
Surface area divided by 1.7 x Adult dose = child�s dose
Comments on Posology 11.8
It is generally acknowledged
that all the methods are flawed, with the surface area rule being the most
accurate. However, it is still flawed. The reason for this, is that the adult
dose is flawed because of individual biological idiosyncrasy. There is no
such thing as an average adult. We live in an age of assembly line medicine.
Standardised pills,
to fit into standardised packets,
to be swallowed by standardised
people. Never overlook the potential
for disaster when compounding. Become familiar with the pharmaceutical
calculations in paragraphs
7.43, through to
7.60.
Remedies in Liquid Form 11.9
In recent years, liquid
remedies for internal use have declined, and have been replaced by the
ubiquitous tablet or capsule, this has much to do with its ease of packaging, a
more precise dosage and convenience of the consumer. In addition, enteric coatings
enable a medication to be introduced to the duodenum,
rather than the stomach.
Nonetheless, this has been at the expense of ease and speed of absorption.
However, there are cases where a liquid remedy is essential, for example, the
very young or elderly patients who have difficulty in swallowing.
There are 3 main groups of the liquid remedies;
1. Emulsions - The Emulsions are either a water in oil, or an oil in water mixture, coupled with an emulsifying agent.
2. Solutions - These are preparations of one or more plant drugs dissolved in a solvent, e.g.,Elixirs, Syrups or Tinctures and Extracts.
3. Suspensions - Are preparations which contain fine mesh powders, which are suspended in a liquid carrier, and will require shaking before use.
The Emulsions 11.10
The emulsions may be used internally or topically
for medicinal or cosmetic
purposes. If the remedy is to be used internally, then the purpose of the
emulsion is to disguise any unpleasant taste, or circumvent any nauseating
effect, for example, Emulsion of Asafoetida. Emulsions for internal use are
rarely encountered today. The major use being as an application to the skin.
Oil and water are immiscible liquids, but a temporary emulsion may be formed by a brisk shaking of the liquids. However, upon standing, the oil and water will once again resolve themselves into two separate layers. There are two types of basic emulsions, they are :
1. Oil in water ( O/W ) 2. Water in oil ( W/O )
The type of emulsion formed is dependent in the relative proportions of oil and water. If water is the predominant proportion, then it will be an O/W emulsion. If oil is predominant, then the emulsion will be W/O.
The predominant liquid is called the Continuous Phase. The lesser proportion is called the Disperse Phase. There are 2 basic requirements to produce an emulsion of immiscible liquids, they are :
1. The disperse phase must be reduced to fine droplets, which are dispersed through the continuous phase.
2. A third agent known as the Emulsifier, which should suspend and prevent the immiscible liquids from Cracking or Creaming. Creaming is the concentration of dispersed globules. Milk with a layer of cream on top, is a good example. Cracking is the destruction of the Emulsion system.
Cracking is the most common problem with emulsions. The change may be biological, chemical or physical. The most common causes are ;
1. Bacterial action, which affects the emulsifier and alters the pH bringing about chemical changes in the phases or the emulsifier.
2. A change in the pH by chemical means which modifies the emulsifier.
3. Extreme temperatures, which changes the properties of the emulsifier.
4. The introduction of substances which are not compatible with the emulsifier.
Types of Emulsions Figure 11.10A
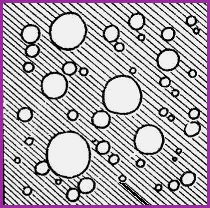 |
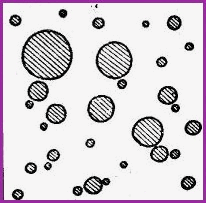 |
|
Oil in Water ( O/W) |
Water in Oil (W/O) |
The shaded areas represent the water phase, and the none shaded areas, the oil phase.
It is a general rule that the continuos phase will determine what type of emulsion is formed.
A further factor in the stability of the emulsion is the type of emulsifier used. Liquid emulsions and light creams are usually oil in water., whereas, the stiffer preparations such as a Balm or Ointment tend to be water in oil.
The Theory of Emulsification 11.11
Emulsifiers depend for their action on the fact that their molecules have a
covalent (non polar) end and an electrovalent (polar) end. A tadpole
would be a good analogy. The head is the polar end, whilst the tail is the non
polar end. The polar head is negatively charged and is Hydrophilic (water
loving) The tail is Hydrophobic (water hating) For convenience, they may
be classified into 4 groups as follows:
1. Anionic Emulsifiers, which form negative ions, e.g., soaps and sulphates.
2. Cationic Emulsifiers, which form cations or positive ions, e.g., ammonium compounds.
3. Non Ionising molecules, such as the esters and ethers.
4. The Natural Colloids, e.g., gums, mucilages, proteins and waxes. This class is of prime importance for the preparation of natural remedies.
Examples of the natural colloids are as follows :
The Gums..
Acacia, which is the dried exudation from the stem and branches of the Acacia spp. Acacia is incompatible with alcohol.
Tragacanth, which is a dried exudation of the Astragalus spp. which is also insoluble in alcohol .
 Mucilages.
Mucilages.
The mucilages are a very useful group of substances, being in most cases both
demulcent and nutritive. In general, they may be used as suspending agents. They
are obtained from both land and marine plants.
Agar, which belongs to the phylum Thallophyta, which comprises the seaweeds and other fresh water forms.
Bladderwrack, Fuscus vesiculosus or Kelp ware.
Chondrus, Chondrus crispus commonly known as Carrageen or Irish Moss.
Cydonia, Cydoniae Semen or Quince seeds.
Fenugreek, Trigonella foenum - graecum L. The mucilage is prepared from the seed.
Ispagula and Psyllium. The seed or husks of the Plantago spp.
Linseed. Linum usitatissimum L.
Althaea or Marshmallow root. Althaea.
Proteins.
As a general rule, these
substances need to be avoided in the preparation of natural remedies. Typical
examples are : Casein, Egg Yolk (lipids) and Gelatine. These
substances are obtained from non plant sources. They are prone to attract
virulent bacteria.
Waxes.
The waxes are obtained from diverse sources, e.g., Bees Animal and Plants.
Chemically they are classed as Esters. Which are large molecules of
alcohol and a fatty acid. They are very variable for common sense reasons, the true waxes
are exudations.
The Fixed Oils and fats 11.12.
The fixed oils and fats are a
mix of the glyceryl esters of high molecular weight aliphatic acids, for
example, oleic, palmitic
and stearic acids.
Chemically they are classed as alcohols, which are representative of a
homologous series. The molecular weights and boiling points display the
usual gradations observed in other series. They are classed as
Primary, which are liquids and are
miscible with water.
Secondary, which are also liquid but are
not totally miscible with water.
Tertiary,
which are greasy solids.
The Alcohols, Structural Examples 11.13.
A molecule of Alcohol contains one or more oxygen atoms in addition to carbon
and hydrogen, for example, ethanol, which is a primary alcohol.
Figure
11.13A.
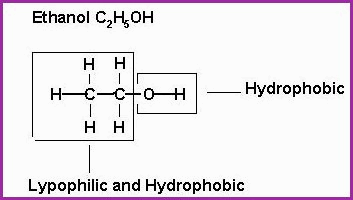
A molecule
of ethanol is hydrophilic (likes water) and hydrophobic (hates
water). It will be seen that the
Hydroxyl group is 1 atom of
Hydrogen short of water (H2O), which explains its affinity for water.
The remainder of the molecule is hydrocarbon, which lends to that part of the molecule Lipophilic (fat loving), and hydrophobic properties. See figure
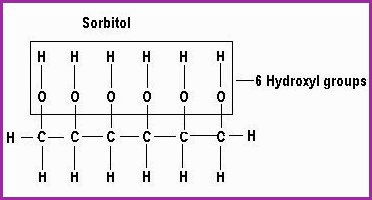
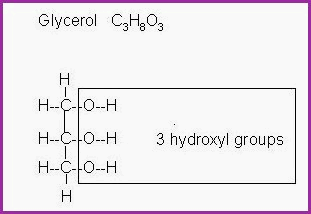
The secondary alcohols are polyhydric, they contain more than one hydroxyl group and are fats and oils. some of the constituents of which, may be used as Humectants. The humectants hold moisture in contact with the skin. They are used extensively in the Cosmetics industry as moisturisers such as Sorbitol and Glycerol. Currently there are some safety questions looming.
|
|
The tertiary alcohols are greasy solids and almost wax like. The hydrocarbon part of the molecule is predominate. Accordingly, it will not dissolve in water. However, if included in a mixture of oil and water, they will act as an emulsifier. For example, Lauryl alcohol.
|
|
Solutions 11.14
The three classes of true
solutions, which are employed in Herbal Pharmacy are as follows:
1. Elixirs ; The elixirs are clear, sweetened solutions of alcohol, water and a medicinal substance. Additionally, a natural flavouring agent is also added.
2. Syrups ; Are sweet concentrated solutions of sugar and water, to which is added a medicinal substance in a suitable form.
3. Tinctures and Liquid Extracts ; This class has been covered in Module 10 They may be considered as the core component of all official herbal medicaments.
A true solution is an ionic or molecular dispersion of a substance in a continuous phase, such as water, alcohol, or a fixed oil. They may be liquid in liquid, or solid in liquid. When preparing a solid in liquid, such as a syrup, the following should be kept in mind;
The sugar is added to the liquid. The solubility of the sugar will increase with an increase in temperature. When the solution has absorbed the maximum amount of sugar possible at a given temperature, it is said to be saturated; Therefore, if the temperature should drop, some of the sugar will precipitate out of solution and re-crystallise. If the solvent of a saturated solution evaporates, it will precipitate out of solution.
Solubility 11.15
The extent to which a solute dissolves
in a solvent, is its solubility. It is usual for national pharmacopoeias to
express solubilities of various substances, as parts by volume of solvent,
in which 1 part by weight (if solid),
or 1 part by volume (if
liquid), of the solute will
dissolve at 15.5 centigrade, unless the temperature is stated differently.
As an example: Gum Acacia is soluble in water 1:1,
whereas, in Alcohol 90%,
it is insoluble. For lesser strength alcohol it will be sparingly
soluble.
True and Colloid Solutions 11.16
The solutions employed in Herbal Pharmacy are either true, or colloidal
in nature. The difference between the two may be summarised as follows; A true
solution is one in which the molecules of the solute are evenly dispersed
throughout the molecules of the solvent. The solution is clear, and is said to
be in the Crystalloid state.
If the molecules of the solute contain electrovalent bonds they will ionise, i.e., they will split into negative and positive ions. If the molecules of the solute remain clumped, the solution will have different properties to that of the true, or crystalloid solution. They will refract light in a different manner, and will be either viscous or gel-like, and are called Colloids.
A simple test to determine what type of solution is formed, is to filter the solution through parchment paper. If the solution is crystalloid, all the particles will pass through. If the solution is colloid, the particles will be retained.
Dispersions and Suspensions 11.17
If an insoluble powder is added
to a liquid and thoroughly agitated, the powder will disperse and form a cloudy
solution. Upon standing, the powder will settle on the bottom of the container
with the clear (supernatant) liquid above it. If it is not possible to stabilise
a suspension by the addition of an emulsifier, then the Signature must
contain the instructions, �Shake well before use�.
Manufacturing Emulsions, Light Creams and Ointments. 11.18
It should be remembered
that Compounding, in spite of the general principles discussed, is more
Art than
Science. The Compounder will have
recourse to many hundreds of different natural substances, which even if of a
similar class, will tend to be of great variability in composition. For this
reason, melting and solidification points are never precise, but are expressed
as a range of temperatures. Theory is subordinate to practice and experience.
The compounder must study the formulation of a preparation and decide whether the preparation is oil in water or water in oil. Separate the fats, oils and waxes and determine the percentage relative to the water and water soluble substances. If the oil phase is the predominate percentage, then the preparation is W/O.
If the water phase is predominate, then the preparation is O/W.
Both types need to be mixed or homogenised in a different manner.
If the preparation is O/W, then a whisk or an electric mixer may be used to emulsify the preparation. A W/O preparation must be stirred slowly, to avoid the formation of air bubbles, which tend to destabilise the emulsion or cream. Due to the similarity of substances employed, it will become obvious that the distinction made between emulsions, creams and ointments, is merely based on the density of each preparation, and in all cases where the preparation is intended for topical use, a suitable preservative or inhibitor of micro-organisms must be incorporated.
Manufacturing an Emulsion 11.19
The proportions of gum and water
to be added to a fixed oil, in order to produce a satisfactory emulsion, will
vary according to the oil used.
As a general rule of thumb,
when using fixed oils, balsams or oleo-resins as a medicinal emulsion, the
following methodology will produce a satisfactory W/O emulsion.
To a clean mortar, add 2 parts of oil to 1 part of a gum emulsifier. Triturate until thoroughly mixed. Then immediately add twice as much distilled water as the gum, and triturate rapidly until the oil has emulsified. Then slowly, whilst triturating, add the rest of the vehicle until the emulsion is complete.
If producing an O/W emulsion, the procedure is modified by first producing a mucilage and adding half the total amount of oil needed to the mucilage, and triturate thoroughly until emulsification is achieved. Then add a further proportion of mucilage, and then the oil until the desired quantity of emulsion is produced. The amount of water used to produce the mucilage will vary according to the substance used to produce it.
Manufacturing Creams and Ointments 11.20
Such preparations fall into three broad categories, as follows;
1. Balm; A balm is an aromatic healing ointment.
2. Salve; A salve is specifically a healing ointment.
3. Cream; Creams are either cosmetic in nature, or used as an emollient.
There are three main functions of such preparations, which are as follows:
1. As an emollient to soften the skin.
2. As a protective surface for the skin.
3. As a vehicle for medicinal substances which are to be used as an application to the skin, or to be absorbed through the skin.
The ointments are fatty substances of such a consistency that they may be easily applied to the skin. The formulation of the ointment base will vary according to the end use and results required. As a product, they are found in great variety and enjoy universal usage by virtue of their versatility.
It should be noted that there are many commercial products which contain glycerine in the formula. Glycerine is contra-indicated for medicinal and cosmetic purposes because of its dehydrating properties. In the case of skin complaints it is injurious and irritating and should be avoided at all costs.
The ointments if kept for long periods, have a tendency to rancidity or attack by micro-organisms. Two readily available inhibitors are Benzoic Acid, which should be added to the preparation in a ratio of 1:1000. Secondly, Salicylic Acid, at a ratio of 1:1000.
The traditional inhibitors and their ratios of usage are as follows;
| Gum Benzoin (Styrax benzoin. Dry.) | 1:100 |
| Popular Buds (Balm of Gilead) | 1:50 |
| Elm Bark (Ulmus rubra. Muhl.) | 1:50 |
The Gum Benzoin and Popular Buds should first be prepared as a 1:1 liquid extract. The extract is then added to the preparation at the ratio given. An alternative method for the Popular buds, is to place the oil or fat into double boiler, and heat. Macerate the popular buds in the warm oil or fat for 8 hours. Strain the resulting liquid and discard the buds.
The Elm bark is of a mucilaginous nature and requires different treatment. The dried bark must first be reduced to a fine powder, and macerated in a fixed oil at a temperature of 40C for 24 hours. The ratio of bark to oil is 1:2. The maceration is then filtered and is used in the end preparation at a ratio of 1:45. When using any of the 5 inhibitors mentioned, be sure to check for any Pharmacological incompatibilities.
Natural microbe inhibitors are all around us in great number. e.g. the essential oils. Check the properties of exeded gums from plant sources in your area, I am sure you will be pleasantly surprised at what is available to you. These natural substances are far more synergistically appealing than the Benzoic acid and of which you may easily prepare oneself.
The Fats, Oils and Waxes employed are of great variety. The choice made, will depend upon the purpose for which the ointment or cream is required. The choice must also include a substance that will remain viable within a given temperature range, which must include body as well as ambient temperature. A selection of such substances and two constants are given in the following table;
| Name | Melting Point | Specific Gravity | Class * |
| Beef Fat (Suet) | 44 - 45 C | 0 . 943 - 0 . 952 | AF |
| Beeswax (Apis - mellifera) | 57 - 64 C | 0 . 953 - 0 . 970 | IW |
| Coconut Oil | 25 - 29 C | 0 . 925 - 0 . 927 | VF |
| Corn Oil (Maize) | 0 . 921 - 0 . 928 | VO | |
| Lard (purified) | 36 - 42C | 0 . 934 - 0 . 938 | AF |
| Myrtle Wax | 45 - 48 C | 0 . 995 - 0 . 997 | VW |
| Olive Oil | 0 . 914 - 0 . 918 | VO | |
| Palm Kernel | 25 - 29 C | 0 . 924 - 0 . 926 | VF |
| Peanut Oil | 0 . 917 - 0 . 926 | VO | |
| Safflower Oil | 0 . 925 - 0 . 928 | VO | |
| Almond Oil | 0 . 914 - 0 . 921 | VO | |
| Sesame oil | 0 . 919 - 0 . 921 | VO | |
| Soya Oil | 0 . 924 - 0 . 927 | VO | |
| Sunflower Oil | 0 . 924 - 0 . 926 | VO | |
| Theobroma (Coco Butter) | 30 - 35 C | 0 . 964 - 0 . 974 | VF |
| Wool Fat (Lanolin) | 40 - 41 C | 0 . 970 - 0 . 973 | AW |
| AF | = | Animal fat............... | Animal fats are absorbed readily by the skin. | |
| AW | = | Animal wax............ | Are readily absorbed when mixed with vegetable oil. | |
| IW | = | Insect Wax ............ | Insect waxes are of minimal absorbency. | |
| VF | = | Vegetable Fat........ | Readily absorbed by the skin. | |
| VO | = | Vegetable oil.......... | Readily absorbed by the skin. | |
| VW | = | Vegetable wax....... | Minimal absorption. |
Library
Pharmageddon
Herbal Block Index
![]()