
 Earth Air Fire and Water
Earth Air Fire and Water
The Pharmageddon Herbal
Essential Concepts of Chemistry
Chapter 7 Part 2
Introduction.
Optical Activity is an inherent power, of biological substances.
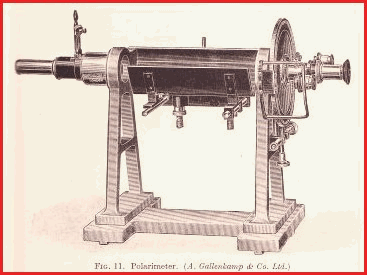
The Polarimeter is simple enough to construct once the principles
have been grasped. It may be made from wood and metal fittings, or other
convenient fabricating material.
The image on the left is a very early model manufactured by a company called Gallenkamp and gives a fair idea of the principles. The analysers of today are considerably more sophisticated and cost a great deal of money.
A natural substance will produce a characteristic rotation signature which is used for identification purposes when examining a sample.
The concept, of a shape of a molecule in space, helps us to understand why synthetic molecules, wreak so much damage and disruption, when taken into a structure composed of natural molecules.
Stereo Chemistry
7.23
In section 7.14, we touched on the subject of isomers, i.e., compounds
that have the same molecular formula but the atoms are arranged in a
different way, which results in differing chemical and physical
properties.
Stereo chemistry deals with the structure of molecules and the position of the molecular atoms in 3 dimensional space. Therefore, we have the further concept of Stereo-isomerism, in which the isomers have the same formula and functional groups, but the atoms are arranged differently in space, which also results in different properties. Stereo isomers govern most of the reactions that occur in a living system.
Structural Representation
7.24
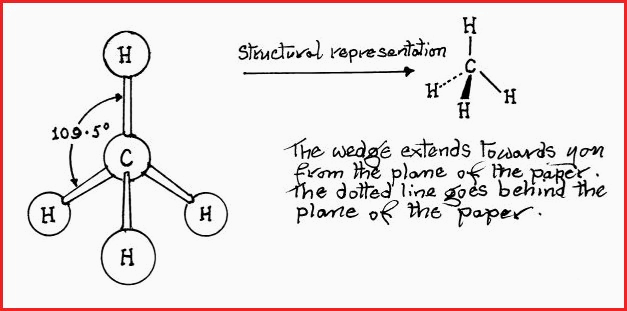
Let us consider a simple molecule of Methane, the molecular formula is CH4.
Molecular geometry is a means of studying the manner in which atoms arrange themselves around a polyvalent central atom, and then the further concept of bond angles. It has already been stated that Carbon is tetravalent, i.e., forms 4 bonds. It has been determined that the Hydrogen bond angles are 109.5� which means that a molecule of methane will have the geometry of a tetrahedron as follows.
Figure 7.24A
All carbon atoms that have 4 single bonds will have a tetrahedral structure, e.g. Chloroform (CHCl3). Most bond angles are constant enough to be considered normal for a particular atom and its state of valence,
1. Water (H2O) Bond angle 105�
2. Ammonia (H3N) Bond angle 107�
3. Hydrogen sulphide (H2S) Bond angle 92�
Molecules and Mirrors 7.25
Mirrors and the reflected object image that we observe are so
commonplace that we rarely stop to think of what it is that we see. There
are two types of mirror image.
1. Those that may be super imposed on each other, e.g.
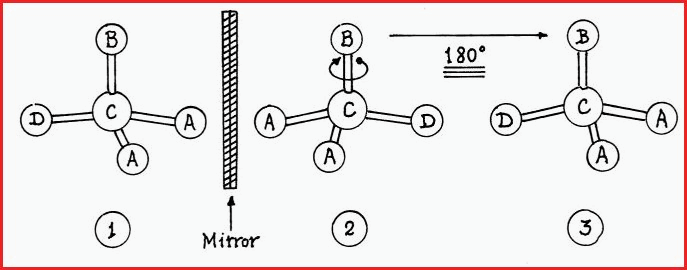
The rules of the game state, that for an object to be super imposable on its mirror image, it is permissible to slide one across the other, such as a circle or a square; or if the mirror image is rotated through 180� and a match is made, then the object is symmetrical.
In Figure 7.25A, molecule 2 is the reflected image of molecule 1. If molecule 2 is rotated through 180� it will have the same geometry as molecule 3. Molecule 3 is then identical to (super imposable) molecule 1. Any molecule that meets that criterion is called an Achiral molecule, irrespective of its structure.
2. Mirror images that cannot be superimposed on each other are said to be Chiral (ky-ral). The word chiral is from the Greek �chier� meaning �hand�. For example, hold your right hand up to a mirror, palm forward, the mirror image that you see is that of the left hand. Compare the palm of your left hand with the image in the mirror. Such structures are asymmetrical or chiral.
Enantiomers 7.26
When the molecular structure of two substances are related to each
other, as in the non super imposable, left and right hands
they are called Enantiomers. The word is compounded from the Greek,
�enantio� meaning �opposite� and �meros� meaning �part�. Molecules the have this left and right hand relationship are called
an Enantiomeric Pair.
Chiral molecules will react in the same manner as Achiral molecules, i.e. Enantiomers will have the same physical and chemical properties, however the rate of reaction differs which gives them different biological properties and they also differ in optical activity.
The �R� and �S� Convention
7.27
The
�R� and �S� convention, is a general method, of
naming the left or right handedness of stereo isomers. The �R�
is taken from the Latin �rectus� meaning �right� or
�clockwise�. The �S� is taken from the Latin �sinister�
meaning �left� or �anticlockwise�.
An added advantage of this system is, that a chemist, by prefixing the name of a compound with an �R� or �S�, can indicate the arrangement, of the four different groups, around a chiral carbon.
To make use of the system the groups around the central carbon are assigned a priority according to their respective atomic numbers. The greater the number, the higher the order of priority.
The atom or group with the lowest priority, i.e. the lowest atomic number, is placed behind the chiral center. The atom or group with the highest priority, i.e. number one is placed at the top. From this position if the next highest priority falls to the right of the chiral center, i.e. a clockwise direction, it is an �R� stereo isomer and if to the left, it is the �S� form,
Figure 7.27A
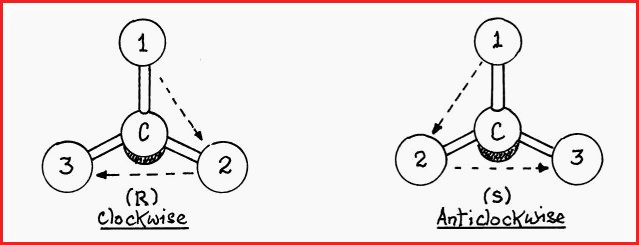
The shaded area represents the atom or group which has the lowest priority
In para.7.26 it was stated that the (R) and (S) forms of an Enantiomeric pair have different rates of reaction and exhibit different biological behavior, for example, it is known that the (S) form of the alkaloid nicotine has a greater toxicity than the (R) form. The (R) and (S) form of some artificial sweeteners is also detectable. A further much quoted example is that of Carvone, which a naturally occurring constituent of many umbelliferous plants is. The (R) and (S) forms have different odors.
Figure 7.27B
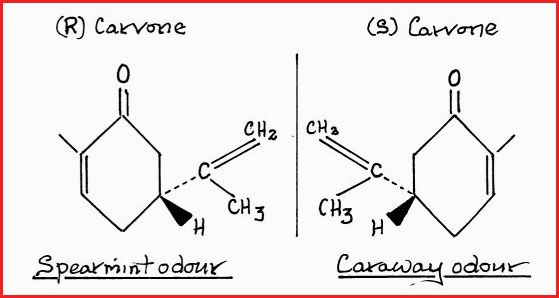
Many enzymes are known to be stereo specific, they will interact with one form and not the other. This fact alone should send shudders down ones spine and it offers a further explanation of the huge increase in disease and mortality of mankind. The plants that we use for food and medicine have balanced R and S structures. The synthetics which have been introduced for flavours cosmetics and medicines do not. Even when the chemist knows which is the preferred form, they are not able to resolve the mix which they have constructed. The havoc and damaged that this has caused is beyond dispute. The synthetic mirror images in many cases passes straight the the walls of the intestines where as the natural substance if harmful would be blocked. The folic acid situation is a case in point.
Optical Activity 7.28
In 1808, a British chemist and physicist W H. Wollaston predicted
molecular geometry. Possibly the insight was stimulated by his
interest in light and crystals, for amongst other things, he invented the reflecting Goniometer which is a device that
measures angles on crystals. At our current state of knowledge we may see
clearly how he arrived at the concept of molecules in three dimensions ..
I wonder what the molecules look like in all of the 7 dimensions?
Not surprisingly, the evidence for molecular geometry started to accumulate in the area of crystallography. Suffice to say, that in 1815 the French physicist J.B. Biot demonstrated that natural organic compounds, whether liquid, or in solution, rotated the plane of polarized light to a greater or lesser degree depending on the substance. Such substances were said to be Optically Active.
Polarised Light 7.29
If light is passed through a suitable filter, light of a uniform
wavelength is produced. The light will vibrate in all planes
from 0 to 360�. If the light is then polarized it will only
vibrate in one plane. A common example of a polarizing filter is that of
the well known Polaroid sunglasses. A simple example of the polarizing of
light follows.
Figure 7.29A
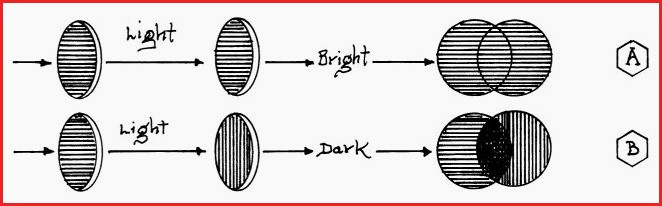
When an optically active substance is placed in the path of a beam of polarized light, the beam will be deflected, or to use the correct term, it will be rotated. The angle of the deflection is noted and it is called the �Observed Rotation�.
The Polarimeter 7.30
The observed rotation of a compound is a physical property which is
used for identification purposes. Chiral molecules which have different
shapes will deflect light in a different plane.
The Polarimeter is the device that is used to study and identify optically active substances.
Fig 7.30A
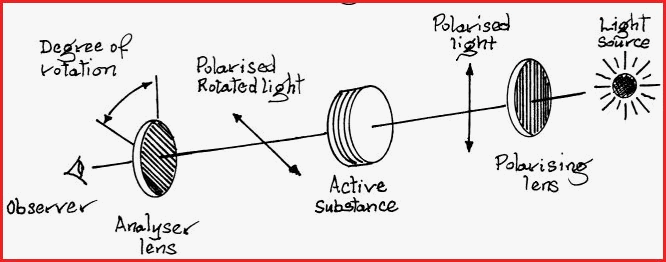
The apparatus is enclosed in a tube which has a port incorporated for the insertion or removal of the substance to be investigated. The Polarimeter must first be calibrated, the procedure is simple. With the Polarimeter empty, the light is turned on and an observation made.
If light is observed the analyzer lens is rotated until the light is blocked and the field of view is dark. The reading is taken from a scale usually attached to the Analyser lens. A number of readings may needed. Especially if the instrument is of an older type. This position of dark field is as represented in figure 7.29A
A liquid sample of the substance to be investigated, commonly called the cell, is inserted into the body of the Polarimeter. If the sample is optically active it will alter the polarization of the light and it will pass through the analyzer lens, as represented in diagram �A� Figure 7.29A.
To ensure accuracy, several readings of the observed rotation must be made. The analyzer lens is rotated until the field of view shows maximum light intensity. From that position the analyzer is rotated until the light is blocked and the field of view is once again dark. If the analyzer must be rotated to the left to restore the dark field, the optically active substance is said to be Levorotatory , and if to the right, Dextrorotatory.
Figure 7.30B
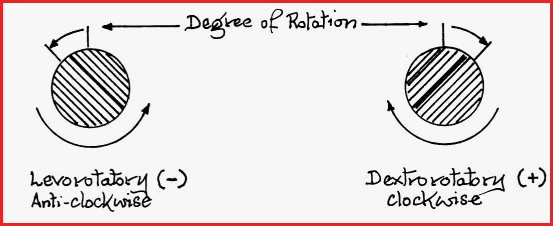
If the substance under examination does not pass light through the analyzer lens, and the field of view remains dark, the substance is said to be Optically Inactive.
The Racemate 7.31
The (R) and (S) forms of chiral molecules are
optically active. However a 50/50 mix of (R) and (S) (+
and -) enantiomers is optically inactive because the optical rotation
of one enantiomer is balanced by the opposite optical rotation of the
other. Such mixtures are called �Racemates�.
The term is from the Latin �rac�mus�, meaning �bunch of grapes�. Racemic acid is an isomeric modification of Tartaric acid which is often found in grape juice. The term Racemisation means the conversion of an optically active substance into an optically inactive substance. Any enzyme which will catalyze the conversion of an optically active into an optically inactive compound is known as a �Racemase�.
Chirality and Biological Systems
7.32
Without doubt chirality is a major factor in all of those reactions
that mediate the essentials of life, in whatever form it may be found.
Generally, nature will only produce from the possible stereo isomers the
one that is the most active, e.g. menthol from oil of peppermint. A further striking example is that of D-glucose, which has 16
possible stereo isomers. D-glucose is a product of photosynthesis in green
plants and is the only one produced from the possible 16.
D-glucose is used to power bodily functions in humans, without it we could not exist. Science does not know how to create optically active substances, although they can propagate chirality by borrowing from nature. However, as stated previously such procedures have led us to the brink of disaster.
Chirality and Synthetic Drugs
7.33
Between them, the Trans-National Pharmaceutical Companies market many
thousands of synthetic compounds on a global basis. Of that figure around
half may be classed as semi-synthetic, i.e. they have a borrowed chiral
structure. Approximately 80% of the semi-synthetic compounds are racemates. The
remaining synthetic compounds are achiral and are not able to undergo �Resolution�,
i.e., the separation of �R� and �S� enantiomers. In many cases it is impossible to �Resolve� a racemic
mixture. Where the resolution is possible, the compound may have to be
routed through 10 or 20 biosynthetic stages to achieve the resolution,
such procedures can be length and also extremely expensive. Consequently
the majority of the pharmaceutical preparations are Racemates. Until adequate safeguards are introduced the chemical and
pharmaceutical industry will continue to market a product on purely
economic criteria which is killing us all.
Lethal Racemates 7.34
When and if the medical historians are finally able to write a
definitive history of the 20th century orthodox medicine, they
will have a burgeoning catalogue of medical disasters upon which to draw. Medicine is �Big� business, and like big business
everywhere, they are supported by cohorts of unscrupulous lawyers, crooked
politicians and renegade scientists, they are often involved in
chicanery, cupidity and stupidity. All of which has been visited on an
unwitting public. The drug companies have labeled their joint products as
�Ethicals�, one cannot help but see the droll cynicism behind
such a name. Especially when the reported fatalities from orthodox
medicine exceed the million mark on an annual basis.
The sort of problems found with the racemates are not confined to the prescription only drugs. The over the counter (OTC�s) drugs and pharmacy only medications (POM�s) are generally considered to be safe and yet, the ubiquitous Aspirin is estimated to be responsible for around 5% of fatal poisonings each year. The much used anti-inflammatory, antacids and laxatives are estimated to account for 20% of all drug related hospital admissions.
The major point to be made is, that many of the drugs implicated in fatalities and extreme reactions had been on the market for a number of years before being withdrawn. Given the evidence surrounding the synthetic and semi-synthetic drugs, the problem must only represent a small percentage of the total.
The Herb as Manufacturing Herbalist
7.35
In discussing chemical structures, we have necessarily had to deal with
the subject, as isolated compounds frozen in time. This is an illusion,
when we look at a living organism, we must understand that everything is
happening at once and at speeds of up to 30 thousand times per second. If we considered the earth as an organ system within a solar organism,
then we may understand that the earth and everything that is on, or in it,
is part of an incredible chemical equilibrium reaction. The synergy of
which is plainly visible on the macroscopic scale, i.e., everything is
connected to everything else. The closeness of the relationship is only a
matter of a degree. The biosynthesis of carbon compounds by a plant is not fully
understood, however by use of radio active markers, and a lot of hard
work, the biosynthetic pathways used by plants have been lightly sketched
in;
Figure 7.35A
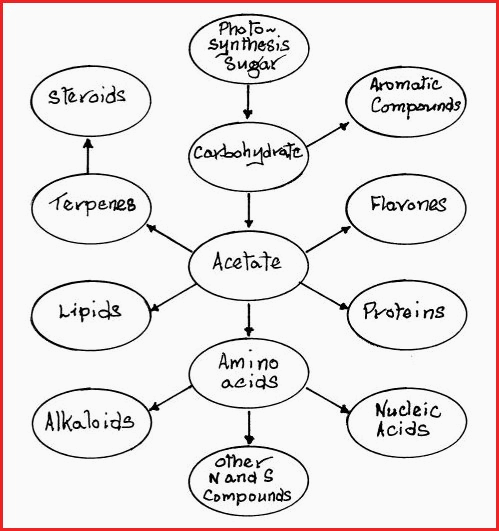
For the purpose of clarity figure 7.35A is a very simply representation. Each division is responsible for many compounds of great complexity. For example, Chlorophyll is the major pigment involved in the photosynthetic process. Its structural representation is as follows;
Figure 7.35B
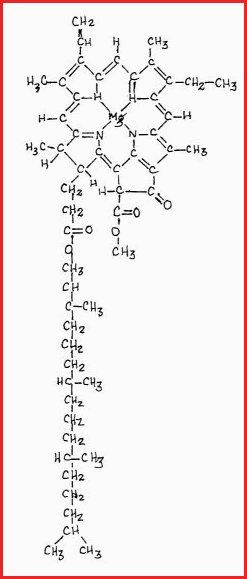
It will be seen that the head of the chlorophyll is a complex ring structure which contains many carbon, carbon double bonds, which are very reactive.
The head is the receptor site for in coming solar energy.
The long carbon tail is embedded, or anchored in a special type of cell called a chloroplast.
Photosynthesis is sometimes described as a reverse respiration process, carbon dioxide is taken in and oxygen is given out. In that respect it is interesting to note the similarity of structure between haemoglobin, which carries oxygen to the tissues of humans, and that of plant chlorophyll. Haemoglobin contains iron (Fe) at the center of its ring structure instead of Mg and contains two extra nitrogen atoms.
From the point when the first leaf shoot of an embryo plant, commence to photosynthesize, a great cascade of biochemical reactions are set in motion supreme alchemy and transmutation at bio temperatures.
At low bio temperatures, and at great speed, many thousands of intricate compounds are constructed. Some are for immediate use and some for storage, while many others are precursors of further synthesis.
Amino acids, enzymes, hormones, nucleic acids, steroids, vitamins, all in great profusion, and unlike the pharmaceutical synthetics that can only provoke a single pharmaceutical response, medications based on the whole plant not only act synergistically, but the action is bolstered with nutritional supplements.
The plant compounds are the same as, or very similar to those found in the human body. In 1970, Lynn Margulis at Boston University, caused an uproar when she advanced the view that the cells of the higher organisms, the eukaryotes, began as communities of prokaryotes, which are single celled organisms. That strongly suggests common ancestry for all life forms, i.e., evolution by division and symbiosis. However, just like the anthropoid theory, it is anybodies guess because we are talking about a timescale of at least 600 million years.
One perceives the fundamental essence of life in the living, not the inanimate, in that which is changing, not in what is finished.
Goethe.
-::-::-
Acids, Bases and Salts 7.36
Acid and base reactions are an important function in all
living systems. Such reactions may occur spontaneously, simply as a result
of mixing reactants. When acids, bases or salts are dissolved in water
they dissociate, the molecules break up and the particles become
ionized. Such particles are called �electrolytes�, because the
solution that contains them will conduct electricity.
Acids 7.37
Acids have a number of chemical properties in common;
1. In the pure form they are non-electrolytes, they do not conduct electricity, if dissolved in water they will.
2. They contain hydrogen atoms.
3. They react with alkali or bases to form salts and water.
4. They will react with carbonates to form salts, carbon dioxide and water.
When a non metallic element combines with oxygen, the oxide formed is usually gaseous. If the gas is combined with water, then an acid is formed.
Bases and Alkalis 7.38
When a metallic element combines with oxygen it forms
an oxide. If an oxide reacts with an acid and forms a salt,
it is called a base. If the base is soluble in water, it is called
an alkali. Alkaline solutions contain hydroxide ions (H3O+).
They are electrolytes and will react with acids to form salts
and water. This fact is of the greatest importance to our interior
economy.
In all things you
shall find everywhere the Acid and the Alcaly.
Otto Tachenius 'Hipporates Chemicus 1670
Figure 7.38A
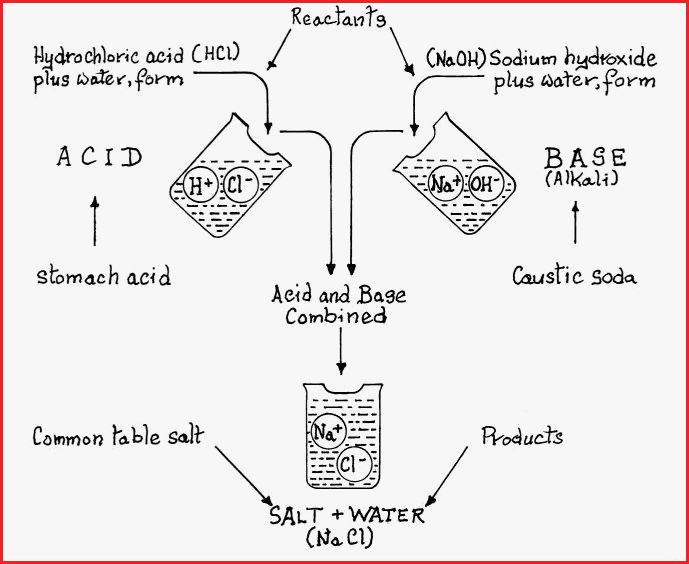
When acid and base are combined in the correct proportion, neutralization occurs and the product is a salt. The type of salt formed will obviously depend upon the type of reactants and the composition of the solvent.
Strong acids and bases result in a fast reaction time; with weak acids and bases, the reaction may be spread over a number of weeks, and therefore go unnoticed, which if alkaloidal salts were being precipitated in plant preparations and where the wrong solvent is used then, it could have serious and even tragic consequences
Buffer Action 7.39
The term buffer is used for certain salts that suppress the
degree of acidity in relation to the actual acid content of a liquid. A
buffer solution, is a solution whose pH is not greatly affected by small
additions of an acid or alkali. There are two types of buffers commonly
used.
1. A weak acid and its corresponding sodium salt.
2. A weak base and its salt with a strong acid.
Acidity and Alkalinity 7.40
A common method to determine whether a substance is acid or alkaline,
is by the use of litmus paper. Litmus is a soluble powder
obtained from lichens. It is also available in solution form. It is
used merely as an indicator of acidity or alkalinity, it will not show the
relative strength of either condition. It indicates the condition by a
change in color. The litmus paper comes in two colors.
1.
Red, which is used to test for an alkali, if the substance is
alkaline the paper will turn blue.
2.
Blue,
which is used to test for acid, if the substance is acidic the paper will
turn red.
If the paper does not change color the substance is neutral (pH7).
To indicate relative acid or alkali strength a universal indicator must be used. Universal indicators are available as paper strips or as solutions. The solutions will give a full spectrum of color changes, from red (acid) through to purple (alkaline). Each kit will contain a color matching chart which indicates the relative strength of the acid or alkaline condition.
The pH Scale 7.41
The acidity or alkalinity of a substance depends upon it concentration
of hydrogen ions. The pH scale is based on the common negative logarithm
the �p� simply means the strength or power of the hydrogen ion
concentration. For example, instead of saying that the pH of a substance is 7, we
could say that the hydrogen ion concentration is 10-7 moles per
litre. However, it is more convenient to use the negative power. The mole
concept will be dealt with later in the text.
Table 7.41A
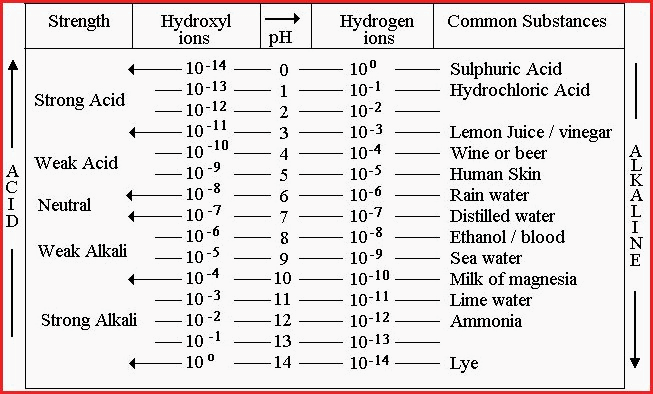
A solution that rates zero on the above scale contains many hydrogen ions (H+) and few hydroxyl ions (OH-), whereas one that rates 14 has many hydroxyl ions and few hydrogen ions.
A pH of 7 is neutral and contains one ten millionth (0.0000001) of a mole of hydrogen ions per litre.
A solution that has more H+ ions than OH - ions, is acid and will be below pH7 on the scale. A movement of 1 whole number on the scale represents a 10 fold change on the previous condition.
Strong acids and alkalis are corrosive and destructive of living tissue. Appropriate precautions should be taken when handling such substances.
Chemical Analysis
7.42
The chemical analysis of a substance involves; firstly the
determination of what is present and secondly the determination of how
much is present. What is present is called �Qualitative�
analysis. How much is present is called �Quantitative�
analysis. We are not much concerned with what is present because in
Herbal work the identity of the natural substances employed will be known. The
quantitative accuracy of the substances used is of prime importance when
compounding or dispensing a medicinal substance.
Quantitative Chemistry 7.43
Quantitative measurements are of two types;
1. Volumetric measurements where the volume (usually gas or liquid) of the substance is expressed in litres or its sub-multiple the millilitre.
2. Gravimetric measurements that may apply to liquids or solids. A substance may be weighed, the unit of measurement is the kilogram or its sub-multiple, the gram.
Alternatively we can determine the density of a substance in which case we refer to it as having mass per unit quantity, i.e. as grams per cubic centimeter ( g/cm� ).
There is a third type of measurement that is concerned with the amount of matter in a substance, i.e., how many entities there are in a given quantity of any substance. The unit of measurement is the �mole�. Its symbol is �mol� and its mass per unit quantity is expressed as mol/L, or kg/mol.
The Mole and Avogadro Constant,
7.44
Molar measurements, are a most useful tool for the
Herbalist. An
understanding of the mole concept will also clarify aspects of homeopathic
pharmacy.
In 1811, the Italian physicist Count Amedeo Avogadro,
published a hypothesis that went largely unnoticed by the scientific
community for nearly 50 years.In brief, the paper suggested that equal volumes of any gas,
under the same pressure and temperature, would contain the same
number of particles. Subsequently, Avogadro�s hypothesis was
developed as a core concept of physics and chemistry. The basic idea is
that individual units are counted by weighing a given quantity, in the
same way that a bank teller will weigh coins to determine their value.
One mole of any substance is its atomic mass number expressed in grams. This amount will contain as many �entities� as there are atoms in 0.012 kg (12 grams) of Carbon 12, which is the standard mole. It will be seen from the Periodic Table, that the elements do not have simple whole numbers, due to the presence of naturally occurring isotopes. It is sufficiently accurate to use the simple whole numbers rounded, e.g. Carbon12, Oxygen16, Nitrogen14 and so on.
12 g of Carbon 12, which is the standard mole, contains;
6.02252 x 1023 atoms.
This number is called �Avogadro�s constant�, or Avogadro�s number. Therefore 1 mole of oxygen weighs 16g and contains ;
6.02252 x 1023 atoms.
Avogadro�s number is astronomical, and when written in full it is quite incomprehensible.
602252 000 000 000 000 000 000.
Avogadro�s number is often cited by orthodoxy to refute the homeopathic claim that the serial dilution of a remedy will increase its potency.
According to Avogadro�s theory, when a serial dilution of 12c (centesimal) or 24x (decimal) has been attained, not a single molecule of the solute will remain in solution. Nonetheless, the enigma of homeopathy remains because undoubtedly high serial dilutions produce a very pronounced effect on a healthy person. Homeopathic pharmacy will be covered later in the text.
Molar Solutions, 7.45
A solution may be defined as a solvent, i.e. a liquid in
which a substance called the �Solute� is dissolved. A
solution is homogeneous.
As stated, 1 mole of any substance is its atomic number expressed in grams, e.g. 1 mole of Hydrogen weighs 1 gram. And contains 6.02252 x 1023 atoms.
1 mol of Oxygen weighs 16 grams. In the case of a molecular compound, the atomic mass numbers are added together; for example water; its molecular formula is H2O and we wish to know the weight of 1 mol H2O, proceed as follows;
Hydrogen atoms = 2 x 1 = 2g = 2 mol �H�
Oxygen atoms = 1 x 16 = 16g = 1 mol �O�
Total = 18g = 1 mol H2O
Therefore, 1 mol H2O weighs 18g and contains
6.02252 x 1023 molecules.
1 mol of any compound is its formula weight in grams. The concentration
of a solute in solution may be expressed in moles per litre (mol/L),
or as grams per litre (g/L). For example, a 1 mol per litre (1mol/L)
Saline solution, would contain 58g of Sodium chloride (NaCl) per
litre, e.g. Na = Sodium = 23g/mol
and Cl = Chlorine = 35g/mol. Total = 58g/mol which is expressed as 1 mole/L or 58g/L
NaCl.
Density 7.46
Density is the measure of compactness or concentration of a
substance and it is defined as its mass per unit volume.
To calculate the density of a substance, the mass (weight) is divided by its volume. If the substance is a solid the answer will be expressed in grams per cubic centimeter (g/cm3). If the substance is a liquid, in grams per millilitre (g/ml).
To calculate the density of a regular shaped solid, first weigh the sample, then calculate its volume. The weight is then divided by the volume. The formula is as follows;
Density = Mass � Volume = g/cm�
For example; a substance weighs 75 grams and its volume is 8.6 cm�, therefore,
75 � 86 = 0.872 g/cm� or s.g. 0.872
If the substance is a liquid, the calculation is the same, except the answer will be in grams per millilitre.
Relative Density 7.47
Relative density is commonly referred to as Specific Gravity or s.g.
Specific gravity has the same numerical value as density, but has no units
such as gram or millilitre. Instead distilled water, at a temperature of 20�C, is used as
the standard, against which the relative density of the other substances
are compared. 1 ml H2O at 20�C will weigh 1 gram. Distilled water
is assigned the value of 1 and expressed as 1.000.
If you refer to Table 6.31A it will show that the weigh per unit quantity, of a given substance (g/cm3) has the same numerical value as relative density or s.g. Accordingly, we may define s.g. as a ratio of the weight of a volume of a substance, compared to an equal volume of distilled water. Or in another way, the number of times that a substance is heavier or lighter that water;
Alcohol 95% weighs 0.810 g/ml and its SG is 0.810 (at 20�)
Water weighs 1 g/ml and its SG is 1.000 (at 20�)
Glycerin BP weighs 1.260 g/ml and its SG is 1.260 (at 20�)
Temperature and Density
7.48
For the Apothecary the s.g. or density, of a given substance is of
prime importance and from it much valuable information is obtained, for
example the alcoholic strength of a solution of alcohol and water may be
determined. If we know the alcoholic strength of a solvent we may calculate the
amount of soluble extractive contained in a given volume. In the first
instance, we can know if the solvent is of the correct strength for
extraction or preservative purposes. In the second, we can know the amount
of solute in the solution.
The density of a substance is a physical property which may be used for identification purposes with the proviso that the measurements, i.e., weight and volume are made at an agreed temperature and pressure.
Standard temperature and pressure (STP) for chemical work is usually 0�C at 1 atmosphere. 0�C, the freezing point of water is not a convenient temperature for routine tasks, which are usually carried out at room temperature. In the older official publications this was defined as 60�F or 15.56�C, however since the introduction of the international SI units the agreed standard is 20�C (68�F).
Metric measurements when given in a Pharmacopoeia are graduated at 20�C so there shall be no errors arising due to the expansion or contraction of solutions at other temperatures. This is particularly important when preparing hydro-alcoholic solvents which are specified for a particular substance.
Considerable heat is evolved when the dilute alcohols are prepared, in addition there may be a disparity between room and solution temperatures. Accordingly, the SG of the alcohols will be shown at 20�/20�. That means the solution at 20�C and the ambient (surrounding) temperature at 20�.
If measuring, by SG, a sample of alcohol and water at 20�C, we determine its alcoholic strength to be 14% by volume at 30�C. The same sample, due to expansion, would only contain 10.75% alcohol by volume. Alcometric and temperature correction tables will be supplied later in the text.
Percentage Solutions 7.49
The term percent is from the medieval Latin �per centum�
meaning �by the 100� and in modern terms the proportion or rate
per 100 parts. The symbol is �%�.In the context of Apothecary work the term �percent� will
have one or four different meanings, only one of which is a true
percentage compound due to differing weights and densities. The meanings
and symbols are as follows;
1. The percentage weight in weight is the only true percentage compound, and it means the weight of solute in a solution, or weight of active ingredient in the product, e.g. 4% w/w of Symphytum extract in an ointment or solutution.
2. The percentage as a volume in volume. This is not a true percentage compound due to the different density and weights of the constituents, e.g. a hydro-alcoholic solvent, Alcohol 30% v/v, which means 30 ml of alcohol in 100 ml of product.
3. The percentage as weight in volume. Again, not a true percentage compound, and it means the weight of active ingredient dissolved in 100 ml of product, e.g. Sodium Chloride 3% w/v.
4. The percentage, volume in weight, meaning the volume as mls of active ingredient in 100 g of product, e.g. Calendula tincture 7.5% v/w which is not a true percentage compound.
Percentage Formulae 7.50
Occasionally formulae may be expressed as �parts�, for example;
Alcohol - 3
Water - 7
This may mean: by volume, or by weight, in either case, the strength of the resulting solution would be different from each other, for example,
Table 7.50A
|
Substance |
Parts |
�C. |
SG |
Weight |
|
Alcohol |
3 |
20� |
0.811 |
0.811 g/ml |
|
Water |
7 |
20� |
1.000 |
1.000 g/ml |
Table 7.50B
|
Substance |
Parts |
By Volume |
By Weight |
As Volume |
|
Alcohol |
3 |
3 ml |
3 g |
3.69 ml |
|
Water |
7 |
7 ml |
7 g |
7.00 ml |
|
TOTALS |
10 Parts |
10 ml |
10 gm |
10.69 mls |
Assuming that the alcohol is the active substance in the solution, then we may say that;
3 as a percentage of 7 is 3 �7 = 42.8% alcohol in the solution by volume.
However, if we have compounded the solution by weight then the percentage of active substance is different, i.e.
3.69 � 7 = 52.7% alcohol. The difference is almost 10%, which is considerable.
Another example would be that the formula may be a combination of liquids and solids as follows;
Starch 1
Salt 10
Water 90
In all such formula the ingredients are weighed, with the exception of the water, because water weighs 1 gram per ml. The rectified formula would read as follows;
Starch 1g = 1% w/v
Salt 10g = 10% w/v
Water 90 ml = 90 ml
This is the method to be followed in the absence of specific instructions. As a further example the formula could be written as follows;
Starch 1% w/v
Salt 10% v/v
Water 90 ml.
It will be understood that formulae �A� and �B� differ in quantities because of the relative density, or s.g. of the substances. If formulation �B� is intended, then it will be as such.
Percentage Composition 7.51
Formulae may sometimes be given by weight and we may wish to prepare a differing quantity to that shown, accordingly, the active ingredient should be determined as a percentage of the total preparation, e.g.
Acetic Acid 25 g
Distilled Water 500 g
In this instance, the acetic acid is the active ingredient and we proceed thus;
25 � 500 x 100 = 5% of the total
As a further example, we may use the saline solution given in Section 7.45, i.e. 1 mole NaCl per litre = Common salt 58g. Distilled water 1000g.
Therefore 58 � 1000 x 100 = 5.8% of the total.
Proportions and ratio�s
7.52
It is common practice to represent drug/solvent proportions as a ratio,
i.e., a given number of milliliters will represent 1 part by weight of the
drug. For example, the general rule for a �Tincture� (There are
exceptions) is that 4 ml of the fluid will represent 1 gram of the drug and
has a ratio of 1 : 4.
I feel this has more to do with convention rather than accuracy and that
w/w would be the better measurement to use.
In the general methods adopted by the Pharmacist, for the
standardization of tinctures and extracts, It is common practice to
represent drug/solvent proportions as a ratio, i.e., a given number of
milliliters will represent 1 part by weight of the drug. In the general methods adopted by the Apothecary for the
standardization of tinctures and extracts, the use of ratios should be
adhered to. Occasionally the formulae of medicinal substances are given in
a variety of ways, e.g. common fractions or decimal fractions, and one
must be able to manipulate the numbers to arrive at a desired.
Occasionally the formulae of medicinal substances are given in a variety of ways, e.g. common fractions or decimal fractions, and one must be able to manipulate the numbers to arrive at a desired term.
The percentage represented by a ratio is arrived at in the following manner, e.g. 1 : 4 = 1 � 4 = 25%.
Decimal Numbers and Fractions
7.53
Decimal numbers have a base of 10. The number may be a whole number or
a decimal fraction. To separate a whole number from a decimal fraction,
the term decimal �point� is used. All numbers to the left of a
decimal point are whole numbers, and to the right, fractions. For any
number less than 1, it is the custom to place a zero to the left of the
decimal point thus avoiding gross error if the point is omitted.
Reading Decimal Numbers 7.54
Table 7.54A
|
Whole Numbers. |
Decimal Numbers. |
Decimal Fractions. |
|
Units |
1 |
Tenths |
|
Tens |
2 |
Hundredths |
|
Hundreds |
3 |
Thousandths |
|
Thousands |
4 |
Ten Thousandths |
|
Ten Thousands |
5 |
Hundred Thousandths |
|
Hundred Thousands |
6 |
Millionths |
|
Millions |
7 |
Ten Millionths |
Multiplying and Dividing Decimal Numbers 7.55
When multiplying a decimal number by units of 10, simply move the decimal point to the right by the number of zeros that there are in the multiplying number, e.g.
0.5 x 10 = 5.0 0.5 x 100 = 50 0.5 x 1000 = 500
When dividing by units of 10, move the decimal point to the left by the number of zeros, e.g.
0.5 � 10 = 0.05 0.5 � 100 = 0.005 0.5 � 1000 = 0.0005
Changing Percentages to Decimal Fractions 7.56
The
denominator of a percentage is always 100. If the
numerator is divided by the denominator the answer is the decimal
fraction, e.g.
5% = 5/100 therefore 5 � 100 =0.05
20% = 20/100 � 20 � 100 = 0.20
43% = 43/100 � 43 � 100 = 0.43
Changing Decimal Fractions to Percentages
7.57
A decimal fraction is converted to percentage simply by moving the
decimal point to the right and substituting the percentage sign, e.g.
0.05 = 0.5% 0.20 = 20% 0.43 = 43%
Changing Percentages to Common Fractions 7.58
Remember that percentage means the proportion, or rate per 100 parts, therefore, when converting a percentage to a common fraction, 100 is always the denominator, and the numerator is the percentage under consideration, for example 5%.
Numerator 5 parts
Denominator 100 parts
The fraction is reduced to its lowest terms, that is when the only common factor between the numerator and denominator is 1, therefore, 5% is 1/20th of 100.
Changing Percentage to Ratio
7.59
The numerator is always the first part of a ratio and the
denominator is the second part, e.g. a 25% solution;
25% = 25/100 = a ratio of 25 : 100
The ratio is then reduced to its lowest terms;
100 � 25 = 1 : 4
Changing Ratios to Percent
7.60
The ratio is first changed to a common fraction, then the fraction to
percent, e.g.
1 : 4 = � x 100 = 25 then add the % sign i.e., 25%.
Library
Pharmageddon
Herbal Block Index
![]()