
 Earth
Air Fire and Water
Earth
Air Fire and Water
The Pharmageddon Herbal
The Essential Distillation
Chapter 6 Part 2
Fractional Distillation 6.14
Fractional distillation is an old
technique employed in the hermetic arts to separate the different components
of a mixture. Separation may also be achieved by simple distillation, but
the technique is discontinuous and can become tedious. Whereas the
fractional technique is a continuous process that conserves time and energy.
Any fermented alcoholic liquid is a soup of bewildering molecular complexity, and when it is distilled then a myriad overlapping phenomena occur. Given the great variety of botanic materials which may be subjected to the fermentation process, understandably the fermentation products will be many, which produce a complex of vapor pressures.
Since water evaporation will occur at all temperatures, then the composition of the vapor arising from the heated liquid will be undergoing constant change, as the lower boiling point components distill off in sequence. In Herbology, the major use to which the technique is put, is for the fractional distillation of ethanol from an alcoholic mixture. However, for more advanced work, fractional distillation under reduced pressure may be used for certain of the perfume oils. Further information will be given under the heading of Essential Oils.
The preparation of ethanol, employing the simple distillation technique, will on condensation produce a liquid which contains some 50 to 60 % water, depending on the care taken. Water evaporates at all temperatures above 0�C and water vapor will be carried over with the ethanol.
The collected distillate must then be redistilled a number of times to free the ethanol from as much of the water as possible. Traditionally the old herbalists would distill the liquid seven times. (Spagyric work) However, by employing a fractional column, high strength ethanol may be produced in a single distillation. The principle involved is shown in the following Figure.
Industrial Fractionating Column. Figure 6.14A
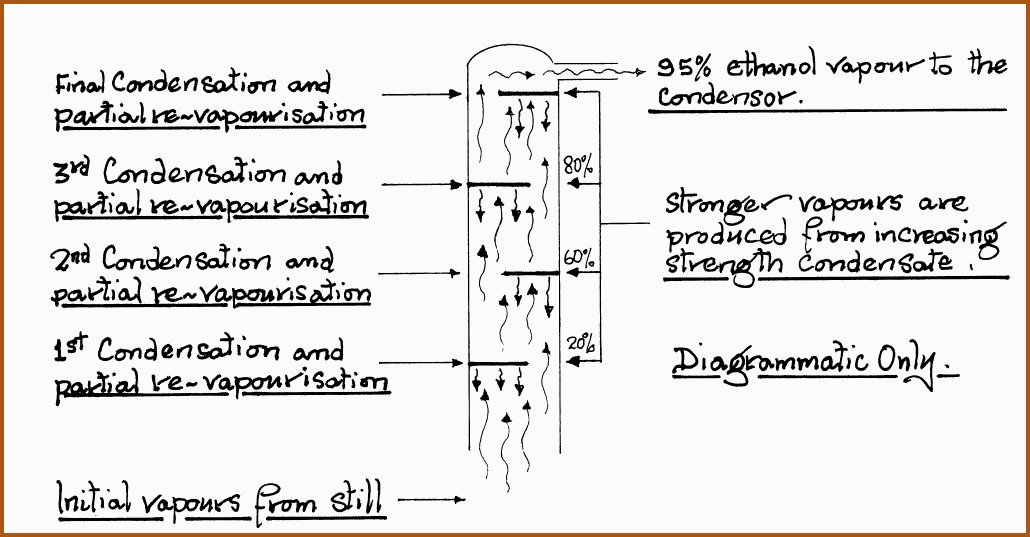
If we have a fermented liquid that contains 10% by
volume of alcohol and we commence to heat the liquid, then both water and
alcohol will start to vaporize. The higher the ascent of the mixed vapor,
the cooler it will become, consequently the vapor with the highest boiling
point (in this case water) will start to condense in the fractionating
column and run back into the body of the still. The vapors of the lower
boiling point continue to ascend the column. In returning to the still, the
condensed liquid will contact the rising vapors and a further heat exchange
takes place, which further enriches the ascending vapor.
Small Scale Fractional Distillation.
Figure
6.14B
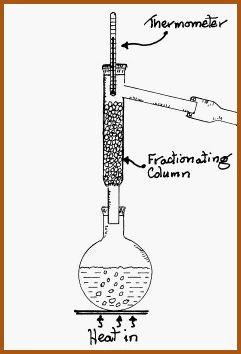 There
are various types of fractionating columns available. One of the cheapest
and reasonably efficient is the so called �Hempel� column, which is
illustrated in Figure 6.14B.
There
are various types of fractionating columns available. One of the cheapest
and reasonably efficient is the so called �Hempel� column, which is
illustrated in Figure 6.14B.
It is essentially a hollow glass tube with a side arm. The tube is usually packed with a suitable material to within 1 cm of the side arm. The packing material should not react with, and allow free passage of the volatile vapours. Suitable material could pieces of glass tubing or small glass beads. If using beads, a small piece of mesh should be placed in the bottom of the column to hold the beads in place. I have found that stainless steel pot scourers cut up work very well.
Fractionating tubes may vary in length. If distilling a liquid where there is a considerable gap between the component boiling points, e.g., alcohol and water, then a relatively short column will suffice. If dealing with a liquid where component boiling points are close together, then a longer tube should be used.
Ideally the temperature of the column should be maintained at some 5 to 10�C below the boiling point of the liquid undergoing fractionation. In large scale equipment, a heating jacket is used. For smaller scale operations the tube may be lagged with cotton wool and held in place with domestic kitchen ties that are normally used to seal plastic bags.
Distillation in Steam 6.15
Steam distillation is a very useful
technique and one that finds wide application in the extraction of essential
oils from plant material. When distilling two immiscible liquids, the
mixture will boil when the sum of the vapor pressure equals the atmospheric
pressure. Accordingly, the mixture will boil at a lower temperature than the
component with the lowest boiling point. Obviously this procedure is sparing
on the delicate nature of the volatile oil molecules.
For example; the boiling point of Carvone at
atmospheric pressure is approximately 230�C, its partial pressure at the
boiling point of water is 9 mm Hg (9 torr). Therefore,
Water at 100�C = 760 mm Hg. Carvone at
100�C = 9 mm Hg. When mixed they boil at 751 mm Hg.
As shown in Figure 4.44A, the boiling point at that pressure approximates 99�C. In relation to the mass of a herb the yield of essential oil is small, therefore, except for analytic purposes, small laboratory scale equipment can be of good service use for small community Apothecary production. In the area of product development small scale equipment is an essential.
The basic laboratory process requires the herbal material to be suitably reduced in size. This will rupture the oil cells of the plant material and release volatiles to the air. To avoid losses the material must be transferred promptly to the flask and then injected with live steam. The volatile oils are then carried over to the condenser with the steam. The evolution of steam may be achieved by employing various methods.
Distillation in Steam 6.15A
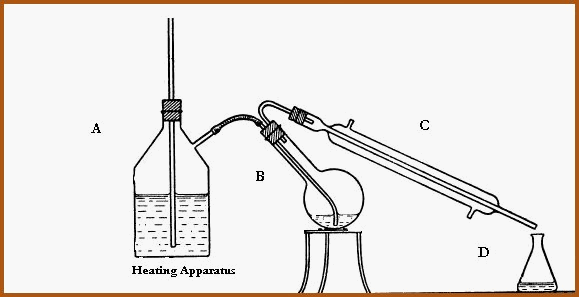
The component marked �A� is the steam generator. The cork or rubber bung is pierced by a hollow glass tube. The tube is the water level indicator. When steam issues from the tube. The heat is turned off and the steam generator may then be topped up. The side arm which is the steam injector may be dispensed with and replaced with a second glass rod through the cork or bung which can then be led to the boiling flask which is at �B�. If it is necessary to introduce heat to the bottom of the boiling flask at �B� then the size reduced herbal material should be partially covered with water lest charring occur. In which case, the distillate will be contaminated with an empyreumatic (burnt) odor, rendering the distillate worthless. The condenser is at �C� and the collecting flask at �D�.
Fabricated Domestic Still (multi-purpose) 6.15B
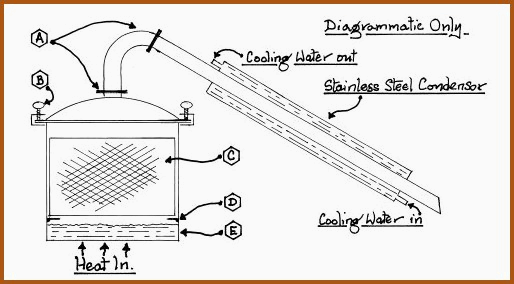
A. Breakable joints for cleaning purposes.
B. Clamps to hold the lid/head secure. The gasket should be made from cork or similar heat and vapor resistant material.
C. Basket made from stainless steel mesh.
D. Welded lugs to support the basket above the water.
E. Water space to generate the steam.
The still, as shown, is very basic but nonetheless versatile. By removing the basket it may be used for simple distillation of water or alcohol. A simple but effective fractionating column may be easily made from stainless steel tubing of a suitable diameter. The liquid level within the still may be determined by measurement of the amount of distillate in the receiver. That amount is subtracted from the original amount of water in the still. The refinements possible will be discussed in Section 6.17.
Distillation under Reduced Pressure 6.16
Distillation under reduced pressure is an
extremely useful technique which has application in 2 major areas.
1. To minimize or prevent decomposition of a thermo-labile substance. When working with heat the thermal cracking of a herb�s molecular structure is an ever present possibility. The subsequent chemical change renders the material unsuitable for the purpose required. Some typical examples of undesirable changes are as follows;
A. Hydrolysis of Alkaloids or Glycosides. Both components are readily decomposed at temperatures exceeding 60�C.
B. All enzymes are inactivated or destroyed at temperatures exceeding 70�C, and in some cases at around 60�C. Fortunately this is a minor problem for the Herbologist, in that very few enzyme active substances are used. A notable exception would be Malt Extract, which must be concentrated at temperatures below 55�C.
C. There are numerous herbal preparations that contain or rely on the presence of Tannins for their medicinal effect, e.g., Hamamelis, Krameria, or Wild Cherry bark. Such plants contain tannins called �phlobatannins�. When heated at temperatures exceeding 60�C Phlobatannins are converted to insoluble compounds, known as phlobaphenes, which are then precipitated from solution.
D. Many alkaloids, when subjected to temperatures exceeding 90�C, will undergo a chemical change known as �Racemization�, whereby an optically active substance may be rendered optically inactive. The new substance will contain the same number and type of atoms but will differ in its structural arrangement, thereby altering or canceling its properties. Such structures are called �Isomers�. Chemical concepts will be covered in the next module.
2. The second major use is to influence the physical form of dry extracts, for the purpose of facilitating the production of granular powders. If a liquid extract is reduced by evaporation to a honey like consistency under atmospheric pressure and then placed under reduced pressure, a sudden evolution of water vapor is induced causing the extract to expand suddenly. The result is a light friable mass which is easily reduced to a granular powder by rubbing through an appropriate sized sieve. The manufacture of extracts will be covered later in the course.
As previously discussed, if atmospheric pressure above a liquid is reduced, then the boiling point of the liquid is also reduced in line with the pressure. Figure 4.44A illustrates the point.
The atmospheric pressure is reduced by means of a vacuum pump. For small scale operations, a suitable pump may be obtained from a laboratory supply company. For production scale operations, an adequate second hand pump may be purchased from a dealer in dairy milking equipment. Such pumps will produce a partial vacuum of 50 kPa or around 350 mm Hg.
The point at which the pump is linked to the apparatus is of importance if equipment is to operate correctly. The following drawing illustrates the usual arrangement of the individual components.
Distillation under Reduced Pressure. Figure 6.16A
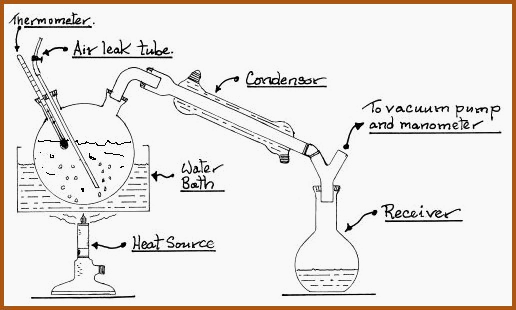
All joints must be airtight. If using ground glass joints, then a very light smear of petroleum jelly will be required.
A liquid, when boiled in a closed flask, will quite often generate a condition known as �Bumping�. Vapor is released in large bubbles instead of a steady stream of smaller ones. That situation is avoided by the introduction of boiling chips, or granules, to the flask. This allows the formation of many small vapor bubbles which are released in a steady stream.
Under reduced pressure the bumping can be very violent accompanied by splashing and frothing of the liquid. In such cases the boiling chips are inadequate. The problem is circumvented by the introduction of an air leak tube to the flask, as illustrated in Figure 6.16A.
The air leak may be constructed of a glass capillary tube fitted with a sleeve of a rubber tube. The rubber tube is sealed with an adjustable clamp. If the bumping commences, the clamp is opened slightly to allow a fine stream of air bubbles through the liquid. This may be done without unduly affecting the vacuum system.
Safety Precautions 6.17
When starting to work with reduced
pressure systems, it is advisable to remember that under a partial vacuum of
0.5 of an atmosphere, that each cm� of the surface of the vessel carries a
weight of 0.5kg, which increases as the pressure reduces. Glass vessels,
unless specially strengthened may implode with all of the consequent
dangers. Glassware for vacuum work may be purchased from laboratory supply
houses. Think about what you do ��
before you do it !
A Simple Vacuum Pump and Manometer 6.18
A simple but effective pump, for small
scale vacuum work, is the water jet pump, sometimes called a �venturi
tube�. The pump is simply connected to a water tap that operates at
mains pressure or around 100 kPa. The water jet pump is quite efficient and
with the proviso that the system is airtight, and the mains pressure remains
constant, then the vacuum attained will correspond to the vapor pressure of
water for a given temperature. The vapor pressures shown in Table 6.18A may
be read in mm Hg or in torr (1 torr =1 mm Hg).
Vapour Pressure of Water. Table 6.18A
|
Temperature �C |
Vapour Pressure |
|
5 |
6.5 |
|
10 |
9.2 |
|
15 |
12.7 |
|
20 |
17.5 |
|
25 |
23.7 |
|
50 |
92.5 |
|
100 |
760 |
Water jet vacuum pumps, are usually constructed of non ferrous metal and sometimes of glass, with varying degrees of sophistication. However, the principle of operation is simple and an adequate pump may be constructed from metal or glass tubing.
Water Jet Vacuum Pump. Figure 6.18A

For various reasons when working with reduced pressure, there is the ever present possibility of contamination, due to suck back caused by leakage in the apparatus. Therefore, it is common practice to place a trap between the pump and the apparatus, so that if the pressure is equalized, contamination will not occur.
A Simple Trap. Figure 6.18B
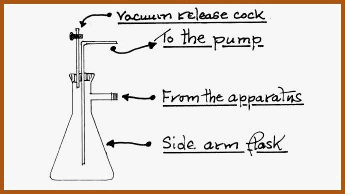
The flask as shown is the �Buchner� or filter flask. The Buchner flask is strengthened to facilitate filtration at reduced pressure.
On completion of the distillation procedure the heat should be turned off and the pressure equalized gently by slowly opening the vacuum release cock on the contamination trap, after which the pump may be shut down.
A useful addition to the laboratory equipment for small scale work is the manometer which is an instrument used to measure the pressure of a liquid, gas or vapor. They are simple and easily constructed.
The Manometer. Figure 6.18C
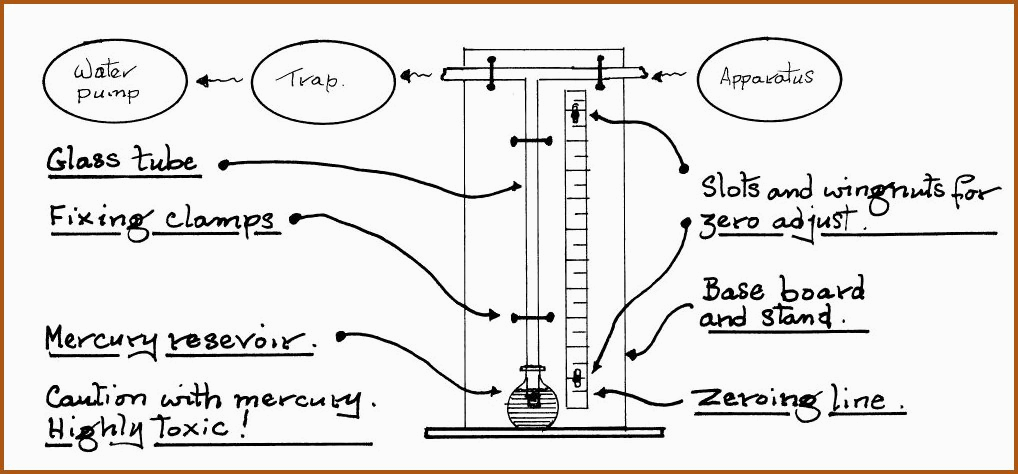
A wooden meter rule is cut at the bottom at the 800 mm line and inverted so that the 760-mm line can be zeroed at the level of mercury in the reservoir. When the apparatus is subjected to a vacuum, the pressure may be read directly from the rule in torr or mm Hg. Ensure that the glass tube and reservoir are of sufficient strength to withstand the reduction in pressure.
For production scale work the manometer is replaced by a vacuum gauge. The herbologist employs low pressure techniques in two areas. The first being in the concentration of the plant extracts and secondly in the production, or separation of essential oils from the material, where that material is of a delicate nature; otherwise, steam distillation is the method used.
In regard to the concentration of extracts, the solvents involved are water and ethanol. Therefore, with the proviso that the apparatus does not leak, then the manometer may be dispensed with. The boiling point (bp) of water and ethanol are known. By comparing the temperature at which boiling commences, with the vapor pressure curves in Figure 4.44A, the degree of vacuum may be known.
Production Scale Equipment 6.19
With consideration given to those points
raised in Sections 6.3 and
6.4, simple or sophisticated production plant may
be manufactured from second hand materials for a modest outlay. As
previously mentioned 2nd hand dairy equipment may be easily
adapted to suit the purpose.
If you can locate a small, owner operated, engineering shop, then the simple diagrams that follow will allow you to dispense with expensive technical drawings. My personal experience of such establishments, has always yielded expert advice and innovative solutions for tight budgets.
When seeking used equipment, the first requirement is for vats and containers. Remember that holes can be patched and fittings removed or modified. Containers and vats may be found from 20 to 4,000 litre capacities. If dealing with liquids, then remember that a batch production will be around 30% less than the nominal tank capacity.
When dealing with a solid, such as fresh or dried herb, then a batch load will be considerably less than the capacity of the tank or vat. As a rough rule for fresh herb; the weight of the herb in kg is circa 12 to 18%, (depending on the material), of the tank capacity in litres. If operating on dehydrated material, then that percentage will increase to 25 to 30%.
The second requirement is for stainless steel pipe and tubing of various diameters. Check to ensure that the bores are not contaminated with scale. If it is, do not be tempted to purchase.
Finally fittings, such as breakable joints, taps, stopcocks, one way valves and sight glasses, will present considerable savings on the price of new.
The text contains sufficient information for you to arrive at a reasonable estimate of water and fuel needs to meet production requirements. Do not be too ambitious and think the whole process through, then commit it to paper, before spending money.
Involve yourself at every stage of plant construction, in that way, you will learn how to service and repair your own plant.
When operating the plant, strict attention to the cleanliness of the equipment must be observed, lest cross contamination occurs. Tubes and pipes must be cleaned with brushes. Spiral coil condensers should have steam passed through them for 30 minutes. For small scale equipment items may be sterilized for 15 minutes in a domestic pressure cooker.
The Multipurpose Still. Figure 6.19A
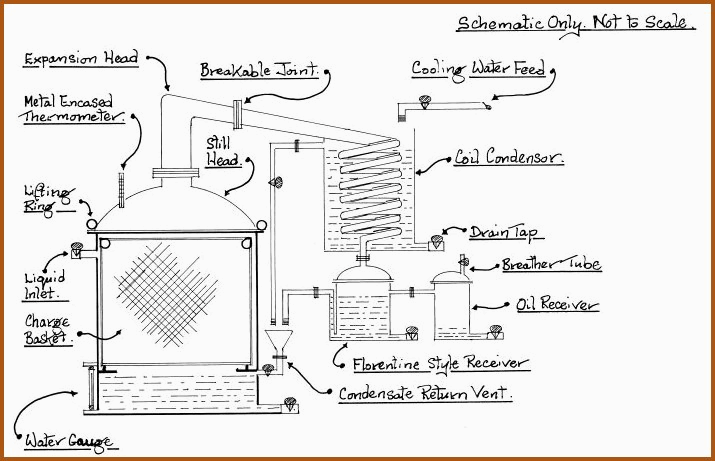
Notes to the Multi-Purpose Still 6.20
The production sized still should take
account of the physics involved and as previously covered. The still as
shown in Figure 6.19A is set up for the steam distillation of essential
oils.
The herb to undergo oil extraction is suspended in the charge basket above boiling water. The steam generated passes though the herb, carrying the volatile oils to the condenser.
The Florentine receiver automatically separates the oil from the condensate. The watery condensate is milky in appearance, which is due to very finely dispersed particles of oil, that it contains. The condensate is also automatically fed back to the still, where it will undergo the distillation cycle again, thereby increasing the overall yield of oil.
For the preparation of distilled water, the charge basket is removed and the condensate return vent is replaced with a constant level device, (see Figure 6.7B). The Florentine receiver is removed and the oil receiver is connected to the condenser.
The arrangement for the ethanol is the same as for distilled water, however, the condensate return vent tap must be turned to the �off� position, to prevent loss of the solvent.
The breather tube on the oil receiver may be used to attach a vacuum pump for any operation that requires low pressure. The still must be made air tight by turning off all inlets that may have contact with the vacuum system.
The still must be thoroughly cleansed after use and if used multipurpose, the following rotation is recommended;
Water - Essential Oils � Ethanol - Water
This will minimize any potential for cross contamination.
For the fractional distillation of ethanol, the expansion head may be packed with stainless steel scourers to within 5 cm of the bend in the head. The expansion head must be adequately lagged (insulated) to prevent undue refluxing of the ethanol. The subject of refluxing will be covered later in the text.
The still may be bottom fired by means of a simple solid fuel block built furnace, upon which the still would stand. A 6mm steel plate must be interposed between the heat source and the still bottom.
The still head and charge basket will need to be removed and replaced with a block and tackle, the still is serviced from a raised platform.
Distillation apparatus is designed for many different purposes in varying degrees of sophistication, with an ascending scale of cost. For pharmaceutical purposes is usually constructed to withstand a steam pressure of 2 kg/cm� (approximately 30psi).
However, such equipment is expensive, and for obvious reasons, steam safety regulations must be complied with. The operator of high pressure steam equipment will, in all probability, require a steam permit.
The Sublimation and the Distillation of Solids
6.21
During the first three decades of the 20th
century, the products of destructive distillation (Pyrolysis) were official
in the pharmacopeias of many nations, e.g., coal tar, birch tar
and pine tar, more commonly known as Stockholm tar. Strictly
speaking such substances are the products of distillation, in that the vapor
phase passes to liquid, rather than directly to a solid.
The solid substance was enclosed, usually, in a cast iron pot, which was then subjected to a high heat that slowly charred the contents. The vapors from the resulting liquids were led away to be condensed on a cool surface, leaving a carbon residue in the pot. The phase changes are represented as follows;
Solid ---> Liquid ---> Vapor ---> Liquid ---> Solid
On further cooling the liquid assumes the familiar semi soft form. True sublimation does not pass through a liquid phase, i.e.
Solid ---> Vapor ---> Solid
The substances evolved from such processes are of little interest to the Herbologist, however, there is another variation on the above, which is as follows;
Solid ---> Liquid ---> Vapor ---> Solid
The process is utilized to sublime benzoic acid from a base of gum benzoin. The benzoic acid is used as a bactericide for the preservation of various herbal preparations. Traditionally powdered gum benzoin or tincture of benzoin, were used for such purposes. Powdered gum is not recommended, due to various contaminants found in the raw gum such as insect parts and bark. The benzoic acid may be conveniently sublimed on the small scale in the following manner.
The Sublimation of Benzoic Acid. Figure 6.21A
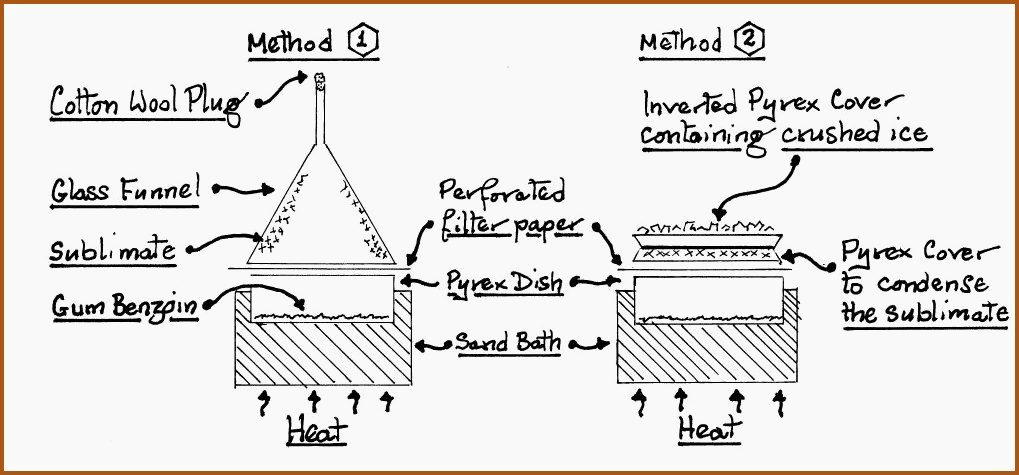
Coarsely powdered gum benzoin is placed in the Pyrex dish; the dish is them covered with perforated filter paper, which serves as a filter trap for unwanted volatiles, this is then topped with the vapor condenser, as illustrated. The sand bath should not be allowed to exceed a temperature of 130�C.
The benzoic acid will form on the surface of the condenser, as white or very pale yellow, shiny crystals. Gum benzoin will melt around 120�C, depending on its origin and degree of purity, and boil at 249�C.
Library
Pharmageddon
Herbal Block Index
![]()