
 Earth
Air Fire and Water
Earth
Air Fire and Water
The Pharmageddon Herbal
Essential Concepts of Chemistry
Chapter 7
Essential Concepts of Chemistry
Laboratorium est Oratorium
[The laboratory is a place of Prayer]
A.L. Mackay.
Introduction.
The painting is by Joseph Wright of Derby.
A Master of Symbolism. Note the full moon, in the top right hand corner.
Many life processes are represented by transmutation, distillation and
fermentation. The Alchemists of yore understood that their work was a
continuation of nature, e.g. Paracelsus stated that the remedy was but a
seed which one must bring to perfection, so it was for good sound
philosophical reasons that they saw their work as sacred.
Chemistry today is very different from its parent. Today is the age of the specialist. Good Generalists are in short supply. Chemistry has many specialist branches. Some of which are ready for pruning. They are producing bad fruit. Nowhere is this truer than where it has its interface with the life sciences. Its manifestation in Pharmacy, Medicine, Surgery and Agriculture is grotesque.
The land, the oceans and the sky vault itself have been severely damaged by this activity. There is no sign of the sacred, or of human respect for life in it. Accordingly, when producing remedies always be mindful of the purpose of your labours.
It is an observable fact, that many human cultures have differing explanations for the same observed phenomenon, and that each explanation, when viewed within its cultural context, provides a satisfactory and logical explanation for what is observed or experienced.
A dictionary definition of culture states that �the ideas, customs and art of a particular society form its culture�. Therefore it is reasonable to say, within the confines of that definition, that a culture equates to a particular mind set.
The global scientific community with its hierarchy, methods and explanations for repeatable phenomena may also be seen as a culture that has no more claim to a transcendent reality than those mind sets labeled magic and religion. In fact, Western scientific technology, if transferred to a former age, would be viewed as magic or supernatural, and science, for all of its intellectual hubris and blind spots, has made us � masters of magic�. Let not a few bad apples spoil what after all can be seen as a magnificent leap of human reasoning power. The ordering of the elements, and with the new scientific nomenclature, they are, in the same manner as the Hermeticists, able to convey their ideas to adepts in any part of the globe.
-::-::-
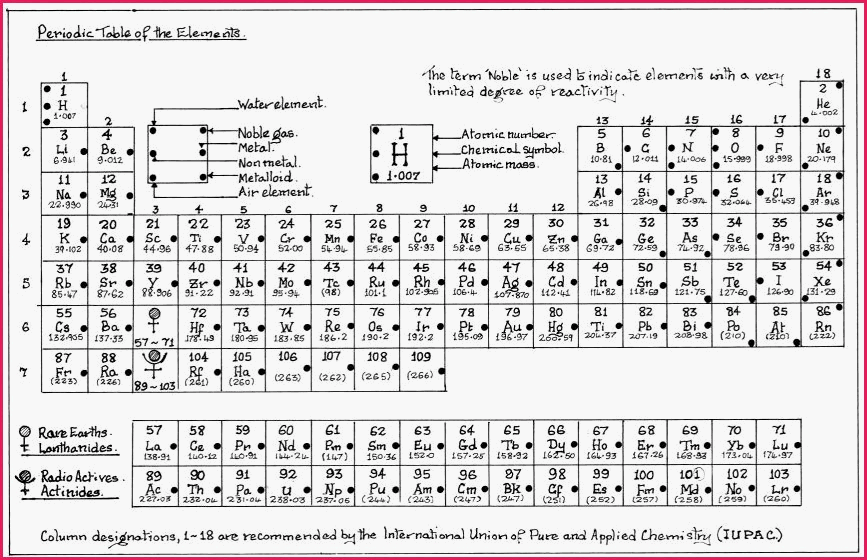
It may be easily understood, that the modern Periodic Table is the fulcrum upon which Chemical Science performs its balancing act. Sir Isaac Newton that exemplar of Mathematics and Physics in a letter to Robert Hooke said;
"If I have seen further, it is
because, I stand upon the shoulders of giants"
Isaac Newton. 5th February
1675.
Newton was an Alchemist.
Thus we come to the eccentric Russian, Dmitri Ivanovich Mendeleyev. The man whose periodic table was adopted over all others. It would be a fair generalization to say that, no great idea ever springs from mans mind fully formed. As an example, Watson, Crick and DNA. All such matters are a metamorphosis of many minds, a braw broth of the zeit geist, the spirit of the times. In 1869 Mendeleyev had his scientific paper read before the Russian Chemical Society. It was entitled � The relation of properties to the atomic weights of the elements�. The zeit geist was at the high point of its manifestation.
Upon whose shoulders did Mendeleyev stand ? Perhaps science would rather not know. In 1864 an Englishman by the name of John Anthony Newlands had a paper published which was to form the basis of his law of Octaves meaning eight. This concept will surface yet again in Chemistry in the �Octet Rule� with regard to valence electrons. Predictably Newlands theory was met with derision from a lot of narrow minded scientific men. However he was later vindicated by the Royal Society. His letter was as follows;
John A. R. Newlands, Chemical News 10,
94-95 20th August 1864.
To the Editor of the Chemical News.
Sir, In addition to the facts stated in my late communication, may I be permitted to observe that if the elements are arranged in the order of their equivalents, calling hydrogen 1, lithium 2, glucinum 3, boron 4, and so on (a separate number being attached to each element having a distinct equivalent of its own, and where two elements happen to have the same equivalent, both being designated by the same number), it will be observed that elements having consecutive numbers frequently either belong to the same group or occupy similar positions in different groups, as in the following examples;
|
Group |
a. |
N |
6 |
P |
13 |
As |
26 |
Sb |
40 |
Bi |
54 |
|
Group |
b. |
O |
7 |
S |
14 |
Se |
27 |
Te |
42 |
Os |
50 |
|
Group |
c. |
Fl |
8 |
Cl |
15 |
Br |
28 |
I |
41 |
||
|
Group |
d. |
Na |
9 |
K |
16 |
Rb |
29 |
Cs |
43 |
Tl |
52 |
|
Group |
e. |
Mg |
10 |
Ca |
17 |
Sr |
30 |
Ba |
44 |
Pb |
53 |
Here the difference between the number of the lowest member of a group and that immediately above it is 7; in other words, the eighth element starting from a given one is a kind of repetition of the first, like the eighth note of an octave in music. The differences between the numbers of the other members of a group are frequently twice as great; thus in the nitrogen group, between N and P there are 7 elements; between P and As, 13; between As and Sb, 14; and between Sb and Bi, 14.
In conclusion, I may remark that just as we have several examples of the apparent existence of triads, the extremities of which are known, whilst their centres are wanting (such as the metals of the platinum group, which may be conceived to be the extremities of three distinct triads, and perhaps also silver and gold may be related to each other in this manner), so we may look upon certain of the elements, e.g., Mn, Fe, Co, Ni, and Cu, as the centres of triads, the extremes of which are at present unknown, or, perhaps, in some cases only unrecognised.
I am, &c. John A. R. Newlands, F.C.S.
Laboratory, 19, Great St. Helens, E.C., August 8.
Newlands hypothesis was not the first, so Mendeleyev arranged his elements in 7 groups, and demonstrated that after each seventh element in a group that their properties repeated themselves. It would appear that even the great Mendeleyev like Newton before him was a Hermetic Philosopher.
Sub Modern Periodic Table.
-::-::-
To consider our current chemical concepts as the final word, is to be blind, because the horizon of knowledge recedes for each step taken. In consideration of chemical theory, it will be understood, that we deal in �electron� chemistry, while all around us at low temperatures nature performs transmutations or �proton� chemistry. In that sense the alchemist, was and is, centuries ahead of time. He is not the deluded fool that is so often portrayed in the text books and populist scientific writing, so much of which, borders on propaganda rather than truth.
In the early 1300�s, an English scholar called William of Occam, stated a principle which is known throughout the scientific community as �Occam�s Razor�, i.e., "entities must not unnecessarily be multiplied", which is now taken to mean, that the simplest theory to fit the facts will be the nearest to reality.
Unfortunately the simplicity or otherwise, of any scientific theory, depends on the size of the body of knowledge to which it applies. Like a tree as it grows from trunk, to branch, to twig becomes ever more complex in form. Thus, the theories become ever more convoluted. A prime example is the 18th century �Phlogiston Theory of Combustion�, which was advanced by the German iatrochemist Georg Ernst Stahl. The theory was supported and elaborated by all of the important chemists of the day, which included Scheele and Priestly. The theory became grotesque, yet just as there is today there was a reluctance on the part of many to say that the theory was a nonsense. Eventually the theory fell before the determined assault of Lavoisier and his �Oxygen Theory�.
The science of chemistry has evolved a system of classifications and protocols, which as a rule will give a high level of predictability with regard to results. Accordingly, its nomenclature is the lingua franca of the global scientific community because it facilitates the precise transmission of a concept from one mind to another.
The purpose of this Chapter is to present an overview of those protocols and the nomenclature of chemistry as they apply to the practicalities of the Herbalists work, thereby avoiding those gross errors of separation and combination which are now so much in evidence in the preparation of natural medicines.
At this stage of growth the market abounds with quack nostrums and spurious systems of natural medicine, which if not exposed, can only lead to the decline of that movement of which we are a part. Whether you compound or prescribe natural medicines you will have the knowledge to judge the efficacy or otherwise of the substances or systems, with which you deal. However let it be said, that orthodox medicine also abounds with quack nostrums and spurious Clinical Trials. Modern Pharmaceutical and Medical remedies are a danger to your life and health. The evidence is overwhelming.
The Chemical Structure of Matter 7.1
In Chapter 4 we dealt with the conceptual
structure of the atom, so let us briefly recapitulate.
An atom may be visualized as a planetary system composed of a nucleus (Sun) which contains two types of particles;
1. Protons which
carry a positive (+) electric charge.
2. Neutrons which have a zero or neutral charge. Each protons (p) carries
1 positive charge.
Orbiting the nucleus in shells or clouds are smaller atomic particles called electrons which carry a negative (-) charge. Each electron carries 1 negative charge.
An atom has an equal number of electrons and protons, therefore its nett electric charge, is zero. There are different types of atoms which are called elements. The elements differ from one another in the number of protons contained in the nucleus. The number of protons contained in the nucleus is the atomic number of the element. The sum total of the protons and neutrons in the nucleus, is its relative atomic mass number.
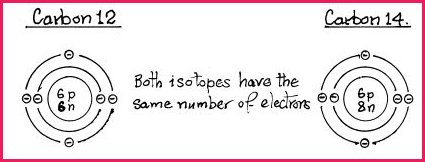
Isotopes 7.2
It is possible to have two or more species
of the same element, i.e., the atomic number is the same but the mass number
is different because the nucleus contains more neutrons than protons. Such
elements are called �Isotopes� which have slightly different
physical properties but will react chemically in a similar manner. Isotopes
of the same element have the same configuration of electrons, e.g.
The nucleus of C12 contains 6 protons and 6 neutrons. Carbon 12 is the most common form found in nature, representing in excess of 90% of all carbon.
The nucleus of C14 contains 6 protons and 8 neutrons, therefore its mass differs from C12. Carbon 14 is radioactive, its physical properties are utilized in biochemistry to determine the fate and excretion of carbon in living systems.
Figure 7.2A
The Bohr Atomic Model 7.3
In Manchester, England, in 1913 the Danish
physicist Neils Bohr was collaborating with the New Zealand physicist
Ernest Rutherford. During that period, Bohr advanced a model of an
atomic structure that has served us well to the present day. Bohr visualized
that electrons orbit the nucleus of an atom in electron shells or different
energy levels which depending on the element, may number from 1 to 7.
Electron Shells 7.4
Remembering that the protons in the
nucleus have a positive charge and the electrons a negative charge, the
electrons are held in orbit by their attraction to the nucleus. A little
thought will make it obvious that the outer most shell or orbital, will need
more energy to hold the electrons in orbit, than will be required by the
inner shells. Therefore, the total energy available to the electrons
circling the nucleus is said to be quantisized.
The 1st which is closest to the nucleus, requires the least energy and is called the ground state, which can only hold a maximum of 2 electrons.
The 2nd level up from the nucleus can hold a maximum of 8 electrons. For atoms that contain 20 or less electrons.
The 3rd energy level can only hold a maximum of 8 electrons or an �Octet�.
Valence Electrons and Bonding 7.5
The highest energy level of an atom, i.e., the shell at the greatest distance from the nucleus, is called its �Valence� level, and all electrons at that level are called �Valence Electrons�. The word is derived from the Latin �valere�, meaning to be strong, and chemically it means the ability of atoms and molecules to bond together and form compounds.
The number of bonds that an atom can make is called its �Valence Number� or valence for short, e.g.
Hydrogen can make 1 bond, its valence is 1.
Oxygen can make 2 bonds, its valence is 2.
Carbon can make 4 bonds, its valence is 4.
Electronic Theory of Valence 7.6
Prior to 1916, it was supposed that atoms
connected to each other by a series of hooks. However during that year the
electronic theory of valence was proposed by an American chemist called Gilbert
Newton Lewis. His theory rests upon three fundamental axioms.
1. That bonding occurs between atoms because each atom strives to achieve a stable electron configuration at its valence level. Further, that stability at the valence level often matched the valence level of the Noble gases. Noble in that sense means to be aloof from or resistant to chemical change, hence stable. The noble gases occupy the 18th group of the Periodic Table. The number of shells for each gas, and the number of electrons in each shell, is shown in the following Table.
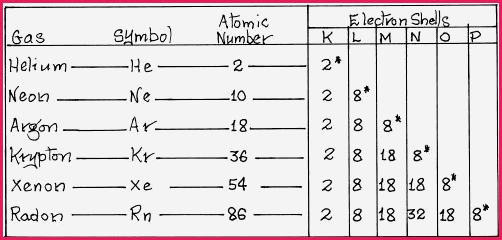
It will be seen in the Table that with the exception of Helium that the valence shells* contain 8 electrons or an �Octet�, which gives rise to a general rule, called the "Octet Rule�. However in chemistry there are always exceptions and other reasonably stable configurations exist.
Most elements do not conform to the valence of the noble gases and they attempt to do so by reacting together chemically to exchange electrons. In doing so an atom may gain or lose electrons. When this occurs the respective atoms are said to form an �Ionic or Electrovalent Bond�.
Generally, when ionic bonds are formed, then atoms with 4 or more, but less than 8 electrons, will gain electrons to achieve stability.
If the atom has less than 4 electrons in its valence shell it will lose them. One of the most common examples of an ionic bond is that of common salt or sodium chloride (Na Cl), as shown in the following diagram, the valence shell of sodium contains 1 electron and that of chlorine 7 electrons

Sodium donates an electron and gains stability because its next lower energy level contains 8 electrons (octet). Chlorine accepts an electron and its valence shell then conforms to the octet rule. A further scrutiny of Figure 7.6A, will show that when the electron transfer has been completed, the sodium atom will have 1 more proton than electrons. Therefore, it becomes Electropositive. Chlorine will have 1 more electron than protons, therefore it becomes Electronegative. When a newly formed particle carries a positive charge or a negative charge it is called an Ion.
An ionic specie is denoted by the charge that it carries, e.g. Na+ and Cl- The sodium and chlorine atoms are bonded together by the molecules electrostatic charge, i.e., the attraction of positive and negative. A negative ion is called an Anion and a positive ion is called a Cation. If an ionic compound is soluble it will dissolve, not into its original atoms, but into anions and cations.
The 3rd axiom is that of Covalency. A stable electron configuration can be attained between 2 atoms by sharing a pair of valence electrons. At this stage it should be made clear that the Hydrogen atom has only 1 proton, 1 electron and 1 energy shell. Its most stable configuration is when it corresponds to that of the noble gas Helium, which has 2 electrons in its valence shell. See Table 7.6A. This is one of the exceptions referred to in axiom 1 and it is very important because Hydrogen plays a major role in bio-structures and when combined with oxygen it gives us water.
The theory of covalence is based on the idea that wherever electrons are to be found in an energy shell, there is a tendency for them to group in pairs. The repulsive effect of 2 negative electrons, is negative because each electron has an opposite spin to that of its partner. As an example, a water molecule has 2 atoms of Hydrogen and 1 atom of Oxygen, and they form a single covalent bond as follows;
Figure 7.6B
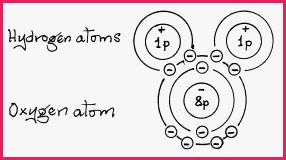
Oxygen has 6 electrons in its valence shell and by sharing a single electron from each Hydrogen atom it attains the stable octet configuration of Neon and likewise the Hydrogen atom attains the stable Helium configuration. The atoms are bonded by their respective electrostatic charges.
Double Covalent Bonds 7.7
A double covalent bond occurs when 2 atoms
share 2 pairs of electrons to achieve a stable octet. A common example is
that of the Oxygen molecule as shown in the following diagram.
Figure 7.7A
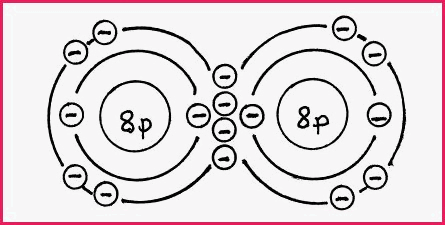
It will be seen from the diagram that the octet requirement has been met.
Polar and Non Polar Covalent Bonds 7.8
When 2 atoms of the same type form a
covalent bond, the bond is considered to be electrically symmetrical because
the electron charge is shared equally, i.e., the average position of the
shared electrons will fall between the two.
Apart from the Bohr model of the atom, there is also an electron cloud model in which the various energy levels are visualized as clouds of energy, which may be spherical or dumbbell shaped, depending on the electrons present in each energy level. Distortions of the cloud are caused by the presence of other atoms. In the following diagram 2 Hydrogen atoms form a covalent bond.
Figure 7.8A

The Hydrogen molecule shown in Figure 7.8A is electrically symmetrical. Symmetrical covalent bonds are Non polar bonds.
If however a covalent bond is formed from atoms of different elements, or if one of the atoms is connected to a 3rd atom, then the molecule will be electrically Asymmetrical, as the greatest electron charge will form close to the atom that has the highest energy level. Such molecules are said to be Polar, for example, a molecule of Hydrogen chloride is polar.
Hydrogen chloride Figure 7.8B
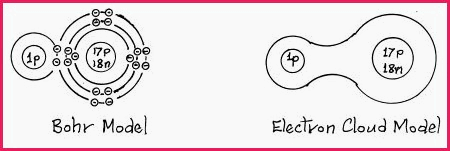
It will be seen that the shared electron pair are strongly attracted to the larger nucleus. The Hydrogen atom has lost some of its electron charge which gives it a weak positive charge, while the Chlorine atom has taken on a negative charge thereby forming a polar covalent bond.
Hydrogen chloride is a colorless gas which when added to water, the reaction forms hydrochloric acid, which is also secreted by the stomach to aid digestion.
The Hydrogen Bond 7.9
Hydrogen bonds play a major role in all
living organisms and are an essential structural feature of nucleic acids,
proteins and starches. Of course they are essential to the formation of
water molecules.
Hydrogen (H) because of its unique structure, will form highly polarized covalent bonds with electronegative atoms, such as Iron (Fe), Nitrogen (N) and Oxygen (O), in the same way as illustrated by the electron cloud in Figure 7.8B. When linked to another atom in a covalent bond, the Hydrogen, with a positive charge, will be attracted to an atom with a negative charge, which in the case of water is Oxygen. The water molecules form Hydrogen bonds with each other in the following manner.
Figure 7.9A
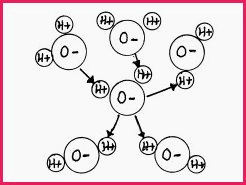
The arrows indicate the direction of the electron attraction. If you study the diagram, it will be understood that the molecules can bond with each other ad-infinitum, which makes possible such large bodies of water as oceans.
Valence and Organic Chemistry 7.10
At this stage it is well to remember that
the valence theory is just a theory, because the valence number of an
element can change according to conditions. (What price William of Ockham?)
Where valence numbers are given, they are to be taken as a common valence,
i.e., the most common isotope of an element.
In 1835, the German Chemist W�hler, in a letter to the Swedish Chemist Berzelius, wrote the following;
"Organic chemistry just now is enough to drive one mad. It gives me an impression of a primeval tropical forest full of the most remarkable things, a monstrous and boundless thicket with no way of escape into which may one dread to enter".
In 1859, and fortunately for W�hler�s sanity, another German chemist, Kekule von Stradonitz, of the benzene ring fame, advanced two postulates;
1. Carbon atoms will bond with each other.
2. Carbon has a valence of 4. (Carbon 12)
As a generalization, this worked well, it allows the organic chemist to make sense of otherwise inexplicable molecular formulae, and most importantly it allows them to explain to each other what it is that they think is happening when a chemical reaction occurs.
The Elements of Life 7.11
Plants, like all biological structures,
exhibit a staggering diversity, that in many cases defies analysis, and yet
this miracle has been constructed from less than 20% of the known elements.
This complexity is merely hinted at by valence theory but it is precisely
the ability of the elemental atoms to bond and form macromolecules that
produces that phenomenon we call life. The following Table lists those
elements most commonly found in living structures.
Table 7.11A
|
------------ |
---------- |
Atomic |
Electron Shells |
|||||
|
Element |
Symbol |
Number |
K |
L |
M |
N |
O |
%by weight |
|
Hydrogen |
H |
1 |
1 |
|
|
|
|
10% |
|
Boron |
B |
5 |
2 |
3 |
|
|
|
Trace |
|
Carbon |
C |
6 |
2 |
4 |
|
|
|
20% |
|
Nitrogen |
N |
7 |
2 |
5 |
|
|
|
3% |
|
Oxygen |
O |
8 |
2 |
6 |
|
|
|
62% |
|
Sodium |
Na |
11 |
2 |
8 |
1 |
|
|
0.10% |
|
Magnesium |
Mg |
12 |
2 |
8 |
2 |
|
|
0.07% |
|
Phosphorus |
P |
15 |
2 |
8 |
5 |
|
|
1.14% |
|
Sulphur |
S |
16 |
2 |
8 |
6 |
|
|
0.14% |
|
Chlorine |
Cl |
17 |
2 |
8 |
7 |
|
|
0.16% |
|
Potassium |
K |
19 |
2 |
8 |
8 |
1 |
|
0.11% |
|
Calcium |
Ca |
20 |
2 |
8 |
8 |
2 |
|
2.5% |
|
Manganese |
Mn |
25 |
2 |
8 |
13 |
2 |
|
Trace |
|
Iron |
Fe |
26 |
2 |
8 |
14 |
2 |
|
0.01% |
|
Cobalt |
Co |
27 |
2 |
8 |
15 |
2 |
|
Trace |
|
Copper |
Cu |
29 |
2 |
8 |
18 |
1 |
|
Trace |
|
Zinc |
Zn |
30 |
2 |
8 |
18 |
2 |
|
Trace |
|
Molybdenum |
Mo |
42 |
2 |
8 |
18 |
13 |
1 |
Trace |
|
Iodine |
I |
53 |
2 |
8 |
18 |
18 |
2 |
0.01% |
The list has been arranged in order of atomic number and is not exhaustive, other elements may be found in living structures. However those given are the major ones found.
Symbols and Formulae 7.12
When two atoms of the same element combine
it is called a molecule. When two or more atoms of different elements
combine it is a compound. In nature an isolated atom is a rarity, the
tendency is for atoms to combine and form larger structures.
Currently there are in excess of 5 million known compounds and that figure increases annually, by the tens of thousands, accordingly, it is essential that the chemist be able to structure and convey information with precision and economy. In a intellectual feat of no mean achievement, they have evolved a symbolic language to meet their requirements.
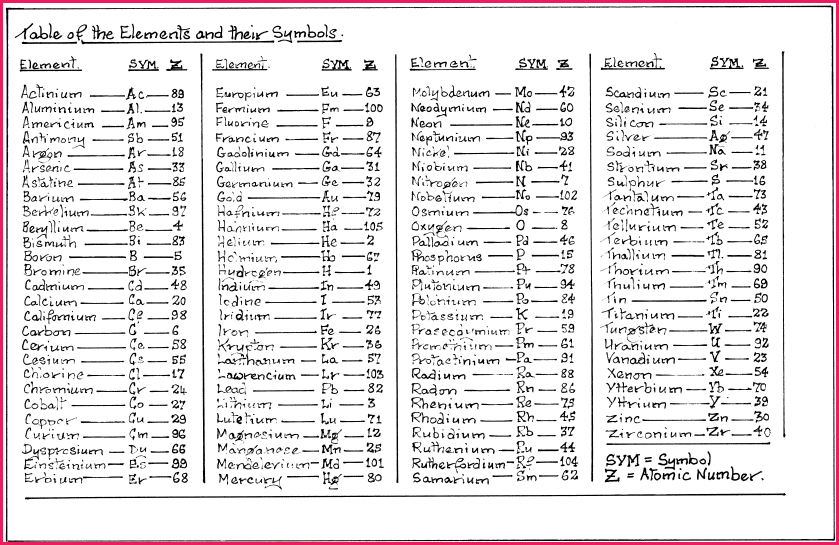
In the early 19th century the Swedish Chemist Berzelius replaced the Alchemical chemical notation used by Dalton, with a system of using letters to denote the elements. To circumvent the problem of there being more known elements than letters in the alphabet, Berzelius added a second letter to a differentiate between those elements whose name began with the same letter, e.g. �C� represents Carbon and �Ca� denotes Calcium.
The names of some of the elements were derived from Latin, e.g. �Au� from aurum meaning gold or �Pb� from the Latin, plumbum meaning lead. Others were named from the mythology or after the person who discovered them. The first letter of an element is always capitalized, while the second is not.
In the language of chemistry the elements may be Monatomic, which means a single atom. As previously stated these are a rarity but do exist and are denoted by its elemental symbol, e.g. �He� (Helium) or Ne (Neon).
More commonly the elements are to be found in the Diatomic form, such as a molecule, for example, Hydrogen, which is denoted with a subscript as follows �H2�. The subscript tells us how many atoms the molecule contains. Oxygen is also a diatomic molecule, and it is written as O2. Strictly speaking, the formula for water is H4O2, however, it is a convention that the molecular formula of a compound is always written as the lowest whole number ratio, which in the case of water is 2:1, i.e., H2O.
Molecular Formulae 7.13
The molecular formula finds widespread use
in the field of inorganic chemistry. The formula is used to describe the
type and number of atoms in a compound, e.g. Sodium chloride = NaCl,
meaning 1 atom of Sodium bonded to 1 atom of Chlorine. See
Figure 7.6A.
Further examples are as follows;
1. Lead chloride, PbCl2 = 1 atom of Lead, 2 atoms of Chlorine.
2. Silver nitrate, AgN03 = 1 atom of silver, 1 atom of nitrogen and 3 atoms of Oxygen.
3. Chlorine, Cl 2 = 2 atoms of chlorine.
Isomers 7.14
Molecular formulae convey precise
information. However they have a limited use in organic or biochemistry
because of the phenomenon of Isomerism. The word is compounded from
the Greek , Isos meaning �equal� and Meros, meaning �part�.
Isomers are compounds that have the same molecular formula, i.e.,
they contain the same types and numbers of atoms but which are clearly
different substances and display different chemical and physical properties,
For example.,
Pentane and Isopentane both contain 5 atoms of carbon and 12 atoms of hydrogen ( C5 H12 )
Ethyl alcohol and Dimethyl Ether both molecules contain 2 atoms of carbon, 6 atoms of hydrogen and 1 atom oxygen the molecular formula is C2 H6 O1 .
The difference in the properties are explained by the way in which the atoms are arranged in the molecule. Isomers exist in great numbers, so it will be clear that a different method must be used to distinguish one isomer from another.
Structural Formulae 7.15
To circumvent the problem of molecular
isomers it becomes necessary to show how the individual atoms are bonded to
each other. There are three different methods, however we will concentrate
on the method that is to be found in all elementary textbooks, in which the
bonds between atoms are indicated by straight lines as follows.
Figure 7.15A
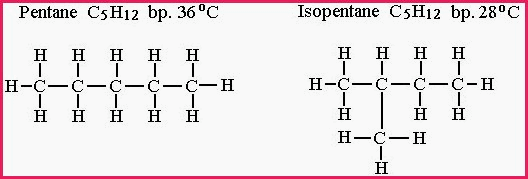
If the carbon and hydrogen atoms in both structures are counted, it shows that the molecular formula is indeed C5 H12 but the chemical and physical properties are clearly different.
Figure 7.15B
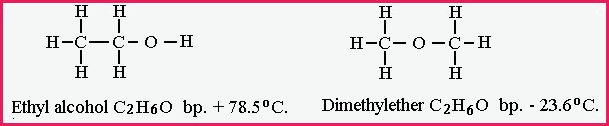
The same situation applies to the isomer C2H6O. The oxygen atom occupies a different place with in each structure . Therefore, the chemical and physical properties depend upon its structural formula rather than the molecular formula. Another common isomer is Glucose and Fructose with a molecular formula of C6H12 O6, however the position of the oxygen and carbon differ in both.
Organic Chemistry and Carbon Compounds
7.16
In the early years of the 19th
century an organic compound was defined as a substance produced by a living
organism. It was believed that such compounds were produced via the
influence of a �Vital Force� which was only present in a living
cell and therefore, could not be synthesized. As an aside here, the
synthetics are lethal if not instantaneously, then over much longer periods
of time so that the insidious action is not recognized. The synthetics cost
less in economic terms but are considerably more expensive in terms of the
biosphere .. Agricultural Chemistry is a prime example
In 1827 the German chemist Friedrich W�hler accidentally synthesized �Urea� (CH4N2O), which is a nitrogenous compound found in the urine of most land based vertebrates. Since that time many generations of children and adults have been taught that the theory of vital force is incorrect and the Vitalism baby was thrown out with the bath water. Take a look around at the damage that this hypothesis has caused. We are on the brink of environmental disaster. And the explanations get ever more convoluted.
Organic chemistry, as distinct from Inorganic chemistry, is a mere 190 years old and is now defined as the � Chemistry of Carbon Compounds�. The rejection of the vital force theory paved the way for the evolution of the synthetic organic chemistry, which in a holistic sense is characterized by the suffering and the extinction of many thousands of living species, this science now it threatens the viability of the planet.
Organic Chemistry and Vitalism 7.17
The vital force theory is a core principle
of all aspects of natural and holistic medicine for some very strong, common
sense and observable reasons.
A living organism at the point of death is made up of the same elements and compounds, and until decomposition sets in, and the metabolism lapses into catabolism, normal chemical reactions continue apace. Yet, quite clearly something has happened, a light has gone out, a chemical switch has turned off, which leaves the enigma of �who or what turned off the switch�? If we subscribe to this line of thought then it becomes obvious that things will become ever more complex and the field of specialists continues to grow. Vitalism is not incompatible with science or its hand maiden chemistry. It is indisputable fact that, for all of the synthetic carbon compounds, not a single one has displayed that vital spark of life.
Characteristics of Carbon Compounds 7.18
1. Carbon compounds may be gaseous, liquid
or solid.
2. They are combustible with a low melting point.
3. Usually they are insoluble in water but soluble in organic solvents.
4. The reaction time is slow but may be measured in a second or less.
5. When they combine the bonds are usually covalent but there are exceptions.
Carbon and Carbon Compounds 7.19
In addition to carbon, organic compounds usually contain Hydrogen
(1 bond) and less often Oxygen (2 bonds) Nitrogen
(3 bonds) Sulphur (2 bonds) and Phosphorus (5
bonds). Occasionally other elements are also found.Carbon, because of
its central position in the Periodic Table, and its valence of 4, is able to
react with many other elements. Most importantly, it is able to react with
one, or many hundreds of other carbon atoms, and form large molecules with
diverse structures that are called macromolecules. When combined with
Hydrogen and/or Oxygen, many thousands of compounds suited to specific
functions are possible. The size of the molecules and their low solubility
in water, make them ideal for the composition of bio-structures. However,
because they are linked by mainly covalent bonds they tend to decompose
easily. As a consequence when manipulating plants for medicinal purposes,
care must be taken and the rules should be followed, lest the synergy of the
compounds be destroyed. Carbon can form double and triple bonds with itself,
and other elements. This it does in a variety of ways.
Figure 7.19A Carbon Bonds
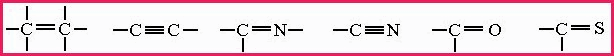
1.Double Bond. 2.Triple Bond. 3.Double Bond. 4.Triple Bond. 5.Double Bond. 6.Double Bond
Classification of Carbon Compounds 7.20
One of the major tasks performed by the
organic chemist is the determination of molecular structures. The carbon
structures have been divided into four main types, which are as follows;
1. The Aliphatic compounds which are Lipids and include the oils, fats and waxes. The word is from Greek �aleiphar� meaning oil. The aliphatic compounds are hydrocarbons and consist of non-cyclic or open ended carbon chains;
Figure 7.20A
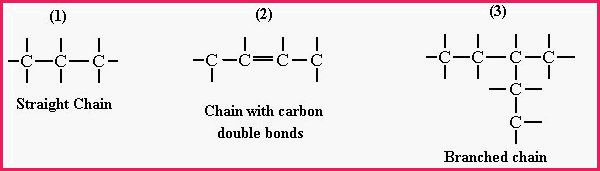
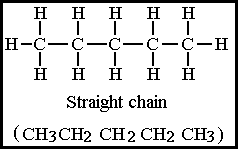
Figure 8.20B Pentane
2. The Alicyclic compounds which contain a ring or cycle of atoms and are analogues of the aliphatic compounds, e.g.
Figure 8.20C Cyclopentane
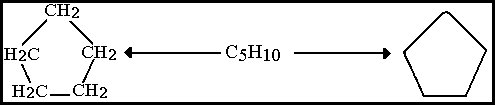
Usual representation, each line segment taken to have a carbon atom at each end.
The Alicyclic compounds are essentially aliphatic in chemical behavior but as may be seen they differ structurally in that the essential carbon atoms are connected as a ring instead of a chain. Compounds that contain a complete carbon ring are known as Carbocyclic compounds. The rings may be in many shapes and sizes with the smallest containing just 3 carbon atoms, however the most common structures are 5 and 6 carbon atoms, but larger structures are also found.
3. The Aromatic compounds include benzene, naphthalene, anthracene and their derivatives, which have an unsaturated ring of carbon atoms. Carbon atoms that have double or triple bonds are said to be Unsaturated because more Hydrogen atoms can be added.
A saturated compound has no double or triple bonds and is unable to undergo an addition reaction. Many of the aromatic compounds have a pleasant odor but some are downright repulsive. A common carbon ring of the aromatic class is benzene
Figure 7.20D
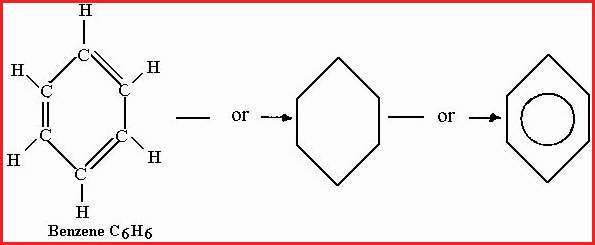
Note the Carbon, Carbon double bonds, the compound is unsaturated. Compounds with more than 1 ring are common. Carbon rings may also contain Carbon chains which have one or more multiple bonds.
4. The fourth class is the Heterocyclic compounds which may be aromatic or Alicyclic compounds in which 1 of the carbon atoms has been replaced by another atom, e.g. Nitrogen, Oxygen or Sulphur.
Figure 7.20E
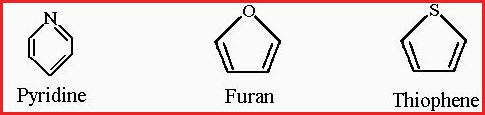
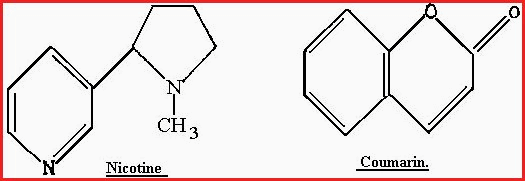
The prefix Hetero is from Greek �heteros�, meaning other, or different. The Heterocyclic compounds are very common and occur in great variety throughout the plant kingdom. For example, the alkaloid Nicotine and the Lactone Coumarin.
Figure 7.20F
Functional Groups 7.21
In the study of molecular structures, it was noticed that different organic compounds tended to take part in similar chemical reactions, they had characteristic properties, in that they behaved in a certain way, irrespective of the other atoms in the molecule.
This observation gave rise to the concept of functional groups. The concept simplified the study of organic chemistry because classes of compounds which contained the same functional group could be studied, instead of the individual compounds.
The term functional group refers to any atom or group of atoms that will substitute for a Hydrogen atom. For example, there are many known organic acids but they all have in common a COOH functional group that release Hydrogen ions (H+) in solution. They belong to the Carboxyl group.
Figure 7.21A

Organic compounds, as often as not, contain more than one functional group and may take part in a number of different reactions depending upon its environment, the surrounding substances, the concentration of those substances, the acid alkaline (Ph) balance and the temperature. The main classes of organic compounds based on functional groups are as follows;
Functional Groups. Figure 7.21B
Figure 7.21Ba Carbon Carbon Bonds.

Figure 7.21Bb Compounds with 1 Oxygen bond.

Figure 7.21Bc Compounds with 2 Oxygen bonds.

Figure 7.21Bd Compounds with 3 Oxygen bonds.

Figure 7.21Be Nitrogen containing compounds.
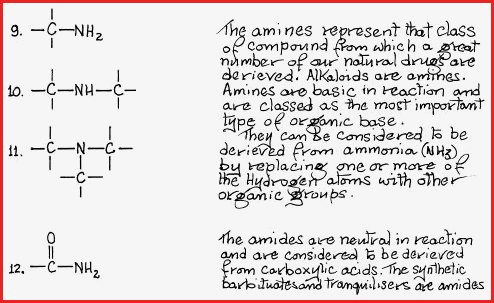
Figure 7.21Bf Sulphur containing compounds.

The functional groups shown are those that contain carbon, oxygen, nitrogen and sulphur atoms which are the most common groups found in nature. There are of course many other known groups to which must be added those which still await discovery.
Simple Chemical Reactions 7.22
At its most simple, a chemical reaction
can be seen as the making and breaking of bonds, a process that requires
energy. In a laboratory that energy is supplied by acids, heat, catalysts
and electrolysis which are crude approximations of the life process.
The known compounds, number in the millions, therefore, the potential for chemical reactions, known and unknown, amongst them is so large, that one would need to calculate in astronomical numbers. Of the small number of known reactions we shall list those of most common occurrence.
Figure 7.22A. Addition Reactions.
Addition reactions occur at carbon-carbon
double bonds and all follow a regular pattern, for example;

This process is used to solidify liquid oils, e.g. margarine. Another general reaction is by the addition of water e.g.
Figure 7.22B
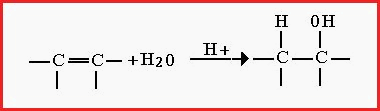
Hydrogen and Oxygen have been shown for the purpose of clarification. Other elements and groups also take part in such reactions.
Combination Reactions.
Combination reactions occur when two or
more substances combine and produce one new substance. The combination may
be of elements, compounds or a combination of the two. The resulting product
is a new compound, e.g.
A + B ---> C
Decomposition Reaction.
A decomposition reaction is the breaking
down of a substance into two or more simpler substances. The original
substance is a compound but the decomposition products may be elements,
compounds or a combination of the two.
C ---> A + B
This type of reaction is sometimes called dissociation.
Single Replacement Reaction.
A + BC ---> B + AC
Single replacement reactions occur when one element replaces or substitutes for another element within a compound. The product is another element and a new compound.
Double Replacement Reactions.
AB + CD ---> AC + BD
This is a common reaction in herbal pharmacy due to the sloppy practice of combining two or more plant drugs or the use of an inappropriate solvent.The reaction occurs when two compounds react together and exchange atoms or groups of atoms. The products of such combining are even more messy. coagulations and murky tinctures or extracts. Which Herbalist can say what his combinations have produced?
Library
Pharmageddon
Herbal Block Index
![]()