
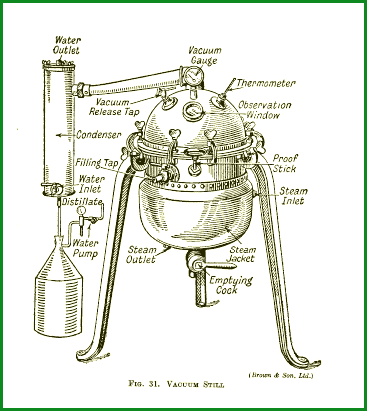
 Earth Air Fire and Water
Earth Air Fire and Water
The Pharmageddon Herbal
Chapter 4 Part 4
PRESSURE
The Measurement of Pressure 4.43
In the mid 17th century many
experiments were undertaken to measure atmospheric pressure, all of which
revolved around Archimedes principle of displacement.
Many liquids were used, e.g. wine and water. The use of such liquids was somewhat cumbersome, i.e. a barometer that used water was some 10 metres in length.
In 1643 Evangelista Torricelli introduced the Mercury (Hg) barometer which measured atmospheric pressure in centimetres or millimetres of Hg.
He demonstrated that 1 atmosphere would support a column of Hg that was 760mm in length (see Fig 4.43A). He is also credited with being the first person to create a sustained vacuum.
That claim by the science historians is dubious,
because some processes that were described in early alchemical or hermetic
literature can only be carried out under a vacuum. However that point is a
digression.
Toricelli Type Barometer
Fig 4.43A
 All pressures lower than atmospheric are
partial vacuums, e.g. at 1500 metres above sea level the barometric pressure
is 87 kPa, which is approximately 653 mm Hg
All pressures lower than atmospheric are
partial vacuums, e.g. at 1500 metres above sea level the barometric pressure
is 87 kPa, which is approximately 653 mm Hg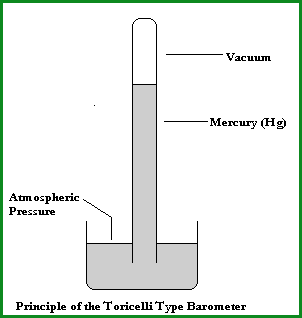
The Herbologist has no reason to operate above
atmospheric pressure, therefore, unless stated otherwise, operations are
carried out at 1 atmosphere and for partial vacuums I will refer to mm Hg.
N.B. In some instances, and depending on
the plant material, when steam distilling for essential oils, it may be
necessary to lift the boiling point of the water employed by a couple of
degrees. This will be explained in the relevant section.
Boiling Points and Partial Vacuum 4.44
Under a pressure of 1 atmosphere, water
will boil at 100 C and alcohol (ethanol) will boil at 78 C. If the pressure
is lowered, then the change of state will occur at a lower temperature, i.e.
the vapour pressure is lowered. If such vapour is then exposed to a higher
pressure, it will condense at a higher temperature than the original liquid.
The relationship between temperature and pressure may be seen from the following vapour pressure curves for water and alcohol.
Vapour Pressure Curves Figure 4.44A
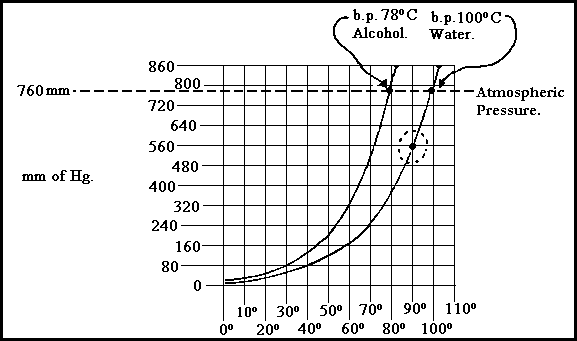
Water beneath a partial vacuum of 560mm Hg will boil at 90�C. It will be seen that as the curves move from a negative to a positive pressure that the boiling point continues to rise. Conversely as the pressure moves from positive to negative, that the boiling point decreases.
The Physics of the Atmosphere and Psychrometry
4.45
Meteorology is the branch of science that
deals with the complexity of gaseous membrane that surrounds the planet. Not
only is the laboratory huge, but the object of investigation is in a state
of constant flux; consequently, the physics of the raw atmosphere can be
difficult to comprehend because of the profusion of phenomena.
Psychrometry is a branch of Meteorology that deals with a given quantity of air in a given volume.
Air is a mixture of gases in different proportions, which will carry a greater or lesser amount of water vapour according to the temperature of the air and the pressure upon it. Therefore, psychrometry is concerned with the physics of dry air and water, ie, enthalpy, specific heat, temperature, vaporisation, condensation, vapour pressure and the amount of moisture that air can carry for a given condition.
It will be understood from the preceding sections that such an analysis would be lengthy and complicated; and that if an immediate decision, such as may be required in dehydration, then the situation is impossible. Therefore, graphs have been developed which when used with a simple instrument, will enable the operator of dehydration apparatus to determine the condition of a given volume of air across a wide range of variables.
Field Condition Temperatures 4.46
To record an accurate temperature of the
ambient air, the thermometer should be set 1 metre above ground level and it
should be hung behind a louvred screen to protect it from direct sunlight.
The screen must allow free circulation of air around the bulb.
Air and Water Vapour 4.47
Air has similar physical properties to
water (refer to sections 4.11 through 4.14). It has temperature, specific
heat and density, therefore it also has weight.
The weight of a given volume of air is determined by its temperature and the pressure upon it, e.g. air will expand with an increase in temperature and its density will decrease with an increase in altitude. Like water, it will also hold another substance in solution or suspension.
Atmospheric air can therefore be considered as a mixture of dry air and water vapour. The presence of water vapour is commonly referred to as humidity. The amount of water vapour that a given volume of air can hold is determined by its physical condition, ie, its temperature, its volume and the amount of water that it already contains.
It has been estimated that the entire surface of our planet transpires some 2 mm of water per day.
Saturation and Relative Humidity 4.48
When air contains the maximum amount of
moisture that it can hold for a given temperature, it is said to be
saturated. That definition will only hold good for free atmospheric air
because in certain processes �super saturation� occurs.
The common measure of proportions in which air and water vapour are mixed, is, relative humidity, which is expressed as a percentage. For practical purposes the percentage is derived from the readings taken from a wet bulb thermometer.
Dew Point 4.49
When dew point occurs inside a dehydration
apparatus, it has a serious consequence for the quality of the herb that is
being dried. Moisture is deposited back onto the the drying herb. Dew point
conditions are a major problem for solar air dryers, in temperate or
sub-tropical zones. The reasons will be expanded in the solar energy
section.
If air has a relative humidity of 100% for its temperature, it is said to be saturated. If a volume of unsaturated air is cooled, and the atmospheric pressure remains the same, the temperature at which its water content will saturate it is known as the dew point temperature.
If the air is cooled below that temperature, the water vapour will condense back to liquid. The greater the temperature drop the greater the amount of water that will be deposited, eg a warm bath and a cold bathroom. When air is saturated, the wet bulb temperature, the dew point temperature and the dry bulb temperature are equal.
If the air is not saturated then the dew point temperature will be below that of the wet bulb. These relationships will become clear when the psychrometric chart is studied.
The Wet and Dry Bulb Thermometer 4.50
There are various instruments in use for
the measurement of relative humidity, e.g. the hydrograph which makes use of
a moisture sensitive substance such as a defatted human hair to activate a
moveable pen which rests on a rotating disc or cylinder, which gives a
continuous read out in graph form. Such sensitive instruments are usually
employed in barometric stations. For field conditions the hygrometers
employed are usually the wet and dry bulb type.
There are 3 types in common use;
1. The aspirator type, where air is drawn across a wet bulb thermometer by means of a hand operated rubber bulb, or alternatively by a battery operated fan.
2. The so called sling psychrometer, this is simply a wet and dry bulb thermometer which is attached to a short chain and handle. The reading is obtained by whirling the instrument through the air.
3. The basic wet and dry bulb type, which is simplicity itself and lends itself to dehydration work. Two identical thermometers are set side by side. The bulb of one thermometer is enclosed in a small muslin sack that tails into a wick. The wick is immersed in a small container of water which is suspended below the wick.
The water climbs the wick and saturates the sack and the enclosed bulb. As the water evaporates from the muslin, it draws heat from the thermometer bulb. Resulting in a temperature drop. The temperature difference between the wet and dry bulb is compared to a set of tables, which gives the relative humidity or degree of saturation, of the ambient air as a percentage. Those tables are incorporated in the psychrometric chart will be studied in due course.
The most accurate thermometer for field conditions is the mercury in glass type. Where possible the water used for the wet bulb reservoir should be distilled and the muslin sack examined on a regular basis for the build up of salts and other impurities which will affect the rate and amount of water diffusion. If this is not carried out then the accuracy of the readings are in doubt.
The wet bulb temperature depends on the evaporative effect. Therefore readings taken in still air or by intermittent air flows will also be inaccurate. The air flow across the wet bulb must be at a constant velocity. Place the hygrometer in the air stream produced by a domestic fan. Two or three minutes exposure should be sufficient to give an accurate reading.
Wet and Dry Bulb Figure 4.50A
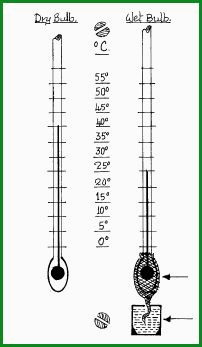 The
diagram shows a dry bulb temperature of 40�C, and a wet bulb temperature of
25�C. This represents a relative humidity of 30%.
The
diagram shows a dry bulb temperature of 40�C, and a wet bulb temperature of
25�C. This represents a relative humidity of 30%.
Wet and dry bulb hygrometers are available in a variety of patterns and sizes according to need. The diagram illustrates the concept.
The wet and dry bulb temperature difference can be translated to a percentage of relative humidity by referring to the Psychometric chart which is shown later in the module.
Muslin Sack.
Water Reservoir.
The Wet Bulb Thermometer 4.51
If the wet and dry bulb temperatures are
equal, then the surrounding air is saturated at that temperature. If the
ambient air is required to take up evaporating moisture, then the dry bulb
temperature must be boosted. The effect is to expand the volume of air by
the addition of heat energy.
The muslin surrounding the wet bulb is saturated with water, therefore when the ambient air passes across it, the air will yield heat to the water and cause it to evaporate. When the water evaporates it will carry the heat with it, this causes a drop in the wet bulb temperature.
At the point when the air leaves the wet bulb, the air is saturated and cannot take up further moisture at that temperature.
The Earth and Dehydrator Climate 4.52
Like an embryo in a uterus the earth is
enclosed in a gaseous membrane, which is collectively called the atmosphere.
It extends nearly 700 kilometres above the earths surface.
The precession of the seasons are caused by a slow wobble on the axis of the earths rotation. Thus light and dark hot and cold, and constantly fluctuating pressures at any given spot on the planets surface will produce a micro peculiarity that we call climate. Fortunately the operator is only concerned with the temperature and moisture content of the raw climate at their location.
The fluctuation of atmospheric temperature will determine how much heat energy must be added to raise it to operational levels. The variation in the moisture content of the raw air, will determine the volume of air at temperature, which must be passed through the dehydrator to satisfactorily dry a given weight of herb.
The amount of air that must be passed in a given length of time will depend upon its velocity. The subject of velocity will be looked at in the section dealing with fans.
If the velocity is too low, it will give and extended drying time, and in extreme cases dew point may occur in the dehydrator. This will affect the quality of the herb.
If the velocity is too high then valuable heat energy will be vented to the atmosphere in a wasteful manner. There is also the possibility that the drying herb will be progressively entrained and blown to the far end of the dehydrator which causes loss and physical damage to the herb.
The amount of heat, and volume of air needed to dry a given weight of herb in a given time, may be quickly determined, by comparison of the wet and dry bulb readings with those shown on the Psychometric chart. The readings are then used to determine the amount of heat and volume of air needed on a batch run basis.
The Fan air velocity is controlled by a variable resistor, similar to those used as domestic light dimmers. Care must be taken to ensure that the control is matched to fan size.
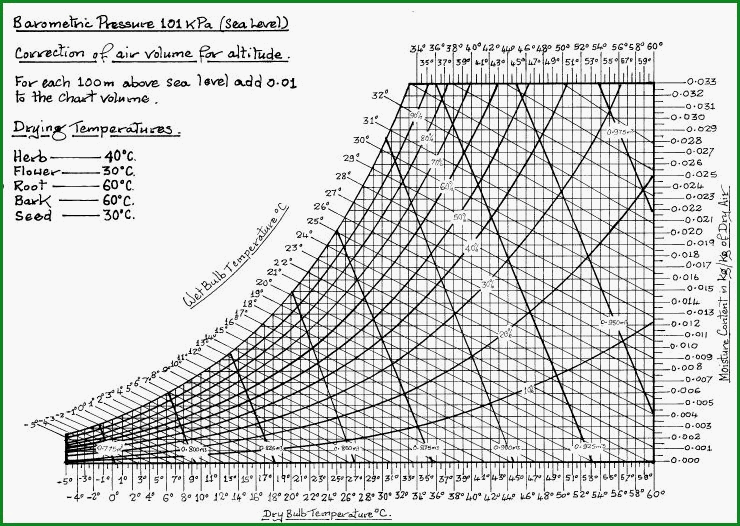
Reading the Psychrometric Chart 4.53
The vapour pressure curves which are given
in Fig 4.44A, allow the user to extract the boiling point of a given liquid
under different pressures. The curves are extrapolated from 2 sets of data
ie pressure and temperature. In the same manner the psychrometric chart
allows the user to read off a range of data which has been extrapolated from
the wet and dry bulb temperatures. It is an indispensable tool. Charts are
available for a wide range of barometric pressures and temperatures. For our
purpose a chart based on 1 atmosphere at sea level will be sufficiently
accurate.
The values given are based on 1 Kg of dry air. Because the volume will alter with temperature, it will be found convenient to calculate in weight of air, then convert to volume as the final figure.
The sectional chart shown in Figure 4.53A is based on ;
1. The raw atmospheric air at the conditions shown.
2. The change in its condition, on being raised to a temperature of 40C, on entry to the dehydration chamber. It may be seen from the chart that the 11C lift in the temperature has created favourable drying conditions. The conditions are maintained by close control of air temperature and velocity.
The operational chart (Figure 4.53B) has been constructed to cover temperatures from minus 5C to plus 60C at 1 atmosphere (101.325 KPa)
Figure 4.53A
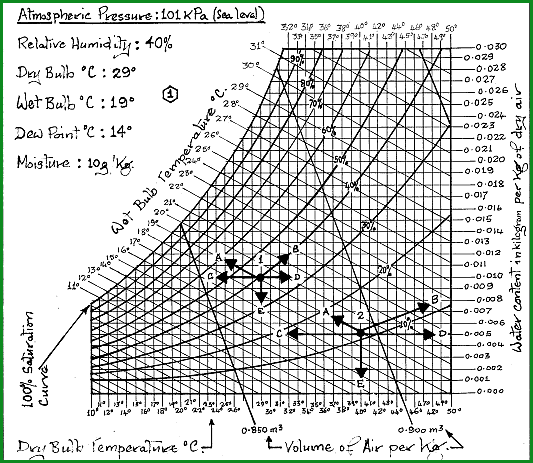
The black dot at the heart of the arrows is the intersection point for all of the readings given. 1kg of dry air at those conditions has a volume of 0.870 m�. For reading 2 the volume of 1kg of dry air is 0.894 m�.
Key to the Chart Table 4.53A
|
Key |
Reading 1 |
Reading 2 |
|
A = Wet Bulb Temperature |
19�C |
19�C |
|
B = Relative Humidity |
40�C |
11�C |
|
C = Dew Point Temperature |
14�C |
7�C |
|
D = Water Content |
10g/kg |
5g/kg |
|
E = Dry Bulb Temperature |
29�C |
40�C |
Psychrometric Chart Figure 4.53B
Dehydration Terms. Selected Glossary 5.54
Absorption. The taking up of moisture from one substance to another.
Adiabatic. The transfer of moisture from one substance to another without an exchange of heat.
Ambient Air. Atmospheric or surrounding air.
Ambient Temperature. The temperature of the atmospheric or surrounding air.
Atmosphere, as Unit of Pressure. 1 atmosphere = 101 kPa or 760 mm Hg.
Boundary Layer. The moisture laden air that forms around fresh or cut herb in absence of air movement.
Bound Moisture. Moisture trapped in the interior of �case hardened� material.
Capillary Flow. The movement of moisture from the interior of the herb to its surface under the influence of heat and air movement.
Case Hardening. The formation of a hard outer layer that resists capillary flow, due to incorrect drying procedures and temperature.
Conditioning. A. The process whereby the dehydrated herb is allowed to reach equilibrium vapour pressure with the ambient air. B. The process whereby dehydrated herb has its moisture content reduced to 5 or 6% to facilitate size reduction.
Constant Rate. The transitional drying period when the uptake of moisture from the herb is at a constant rate.
Counter Flow. The reversal of the air flow across the herb. On entry to the dehydrator, the air contains more heat and less moisture than on exit. It is sometimes necessary, due to the high moisture content of herb harvested after periods of rain or because of wet ambient conditions to periodically reverse the air flow to obtain satisfactory results.
Critical Moisture Content. The mid point between the constant rate of the herb and its falling rate.
Cross Flow. The air is directed at right angles to the herbs direction of travel. Unless a means of recirculating the air is available, then this method is not recommended.
Cyclone Separator. This is a cone shaped device normally used in conjunction with a grinding or milling machine for size reduction of the herbal material. The herb particles fall to the bottom of the cyclone and excess air, after its velocity has been reduced, leaves at the top of the cyclone.
Dehydration. Drying or water removal by controlled air velocity and heat.
Dew Point. The temperature level at which moist air will become saturated and the moisture condense with a small drop in the temperature.
Dry Bulb Temperature. An ordinary temperature reading.
Drying Ratio. The amount of fresh herb required to produce 1 kg of dried herb.
Equilibrium Moisture. The moisture content of the herb after it has undergone �conditioning� process
Falling Rate. Follows the critical rate of drying and as the term implies the amount of moisture removed from the herb steadily declines.
Free Moisture. The unobstructed capillary flow of moisture from the herb, or the evaporation of moisture from a free water surface.
Hydrometer. An instrument used to measure the humidity present in the air.
Hygroscopic. A substance which attracts water molecules.
Labile. Unstable and prone to irreversible change.
Leaching. Loss of chlorophyll or colour due to natural or artificial light.
Maillard Reaction. A group of undesirable changes because of reactions between sugars and amino acids.
Migration. The movement from one place to another of the solutes in the wet or drying herb.
Morphology. In pharmacognosy, the study of the micro and macro forms and structures of plants.
Organoleptic. Sight, touch, taste, smell. Changes in the herbal material which is detectable by the senses as listed.
Oxidation. Undesirable chemical changes which are produced by reaction of damaged herb and atmospheric oxygen. There are similarities to the Maillard Reaction.
Parallel Flow. Usually a dehydrator in which the herbal material and the air flow move in the same direction.
Phase Transition. A change of state, i.e. a solid melting or a liquid vaporising or the reverse order.
Plenum. A large cavity or space which is used as a reservoir for heated air and is commonly referred to as a �heat plenum�.
Psychrometer. A wet and dry bulb thermometer.
Psychrometric Chart. A two coordinate diagram which allows the user to understand the air condition, before and after entry, to the dehydrator heat plenum.
Recirculation An arrangement of louvres and air ducts that allows the air to be partially or wholly re-used within the dehydrator.
Residence Time. The herbal materials exposure time in the dehydrator under operational conditions.
Saturation. As applied to air condition, the point at which air contains the maximum amount of water vapour that it can hold at that temperature and pressure. (See Dew point)
Temperature Kelvin or Absolute. The absolute temperature can be expressed in � Celsius by adding 273 for all temperatures above zero, and subtracting 273 for temperatures below zero.
Thermal Conductivity. The amount of heat energy that can be gained or lost by a substance in a given period of time. The heat conductivity varies from substance to substance. It is important when selecting materials for dehydrator construction because it may have considerable economic repercussions on the operation.
Trolley. A small wheeled rack which holds drying trays at spaced intervals, on which herbal material is progressed through a dryer.
Tunnel Dryer. A tunnel through which warm air is passed to effect the drying process. For the herb processor this type of dryer cannot be matched for ease, speed, economy and quality.
Wet Bulb Depression. The temperature difference between the wet and dry bulb temperatures.
End. Chapter 4.
Library
Pharmageddon
Herbal Block Index
![]()