
 Earth
Air Fire and Water
Earth
Air Fire and Water
The Pharmageddon Herbal
Chapter 6
The Essential Distillation
Introduction.
The distiller�s pot goes back to the
cradles of civilisation. Modern Scholars have expressed puzzlement about the
obvious antiquity of the methods and means, yet there was little mention of
alcohol until the leakage of Arabic Science into Europe during the 12th
Century AD. Perhaps it was because the substance was considered sacred. The
Essentia, the Mercury, the soul of the plant.
Distillation is a means of obtaining a chemically pure substance. A means of separating the wanted from the unwanted.
We may be sure that those ancients probed the mysteries of all substances known to them. Wine would have been no exception.
The distillation apparatus is an ingenious method of emulating the planetary respiration and precipitation cycle. Symbolically it is the manipulation of Earth Air Fire and Water.
In the mundane sense it is an indispensable tool. Which by the use of measurement we may produce phenomena on demand, but within limits. This is the work of a technician and not of a master.The Herbologist makes use of 4 major processes, they are;
1. Fermentation - Chemical action by yeasts.
2. Sublimation - Distillation of a solid, e.g. Stockholm Tar or Benzoin.
3. Calcination - Recovery of salts from extracted residues.
4. Distillation � Separation and purification
The principles and techniques involved in the operations remain unaltered by scale; If you are not familiar with laboratory equipment and safety procedures, then you are advised to obtain an appropriate manual from your local library. Alternatively laboratory safety manuals are available for down load from the internet.
Measurements 6.1
Success or failure, safe or toxic,
effective or useless, are all determined by measurement, accurate or
otherwise. The measurements involved are, volume for liquids, weight for
solids and temperature for heat, kPa for pressure.
Domestic measuring devices are prone to gross error. For calibration purposes having a piece of equipment of known accuracy to which other items may be referred is essential.
Due to multiple factors, even the most sensitive of devices will have an area of uncertainty, e.g., the expansion and contraction of volumetric devices due to temperature. Manufacturers of laboratory equipment will state what the area of uncertainty is, and heat is involved, and at what temperature the tolerance holds good. If weight is involved, at what barometric pressure. The temperature is usually 20�C and barometric pressure of 101 kPa or 760mm Hg.
Average Uncertainty Factors. Table 6.1A
|
Instrument |
Uncertainty |
|
Thermometer Mercury in Glass - 10 to 110�C |
� 0.2�C |
|
Platform Balance - Weight of Solids |
� 0.50 g |
|
Triple Beam Balance |
� 0.01 g |
|
Graduated Cylinder � Liquid - 100 ml |
� 0.20ml |
|
Graduated Cylinder 50 ml |
� 0.20 ml |
|
Graduated Cylinder 25 ml |
� 0.10 ml |
|
Pipette (liquid) 25 ml |
� 0.02 ml |
|
Pipette 10 ml |
� 0.01 ml |
Glassware 6.2
Standard laboratory glassware is available
in two grades, borosilicate or soda glass. Borosilicate is the
better quality, and has greater resistance to thermal shock, which is caused
by rapid expansion or contraction, on heating or cooling. If domestic items
are used, ensure that they are heat resistant. Apparatus for specific tasks
may be constructed by using glass tube and cork, or rubber stoppers. If that
method is adopted, then a gas burner will be needed for the heating and
bending of the glass tube, plus a set of cork borers.
The other option which is to be preferred, is to purchase glassware in sets with ground glass connections. Although the initial cost is higher, over a period of time it will prove to be the best economic option. Contamination problems associated with the cork and rubber stoppers are eliminated. Cleaning is facilitated and the apparatus is quickly and easily assembled or dismantled.
Suppliers will provide a catalogue on request. Ground glass necks and joints are available in a number of sizes. Ensure that all parts match. Each size has a code number, the first part of which designates the diameter of the large end in millimeters, and the second gives the length of the joint. Table 6.2A is representative of the most popular sizes and should be available ex-stock.
Standard Joints. Table 6.2A
|
American |
British |
Size Code |
|
10/18 |
10/19 |
0 |
|
14/20 |
14/23 |
1 |
|
19/22 |
19/26 |
2 |
|
24/25 |
24/29 |
3 |
|
29/26 |
29/32 |
4 |
|
34/28 |
34/35 |
5 |
|
40/35 |
40/38 |
6 |
|
45/50 |
45/40 |
7 |
|
50/50 |
50/42 |
8 |
|
55/50 |
55/44 |
9 |
Figure 6.2A Ground Glass Joints.
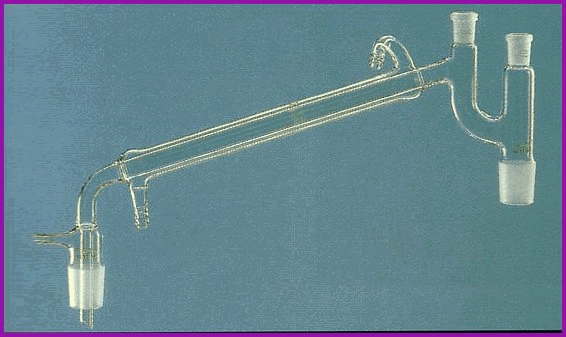
Ground glass joints must be kept scrupulously clean. If working with heat it is advisable to very lightly grease the joints with laboratory grade grease which has been specially formulated for that purpose.
Construction points for small scale plant.
6.3
When considering the economics of small scale processing plant, there are
four factors that need to be balanced.
1. Construction Cost - The costs may be reduced by utilizing used dairy or food processing equipment. The main cost is for welding services.
2. Operational Cost - Energy use is a prime cost. Unwanted heat loss will add considerably to energy use, as will bad design. Faulty design produces increased labor costs, e.g., difficult load/unload procedures.
3. Maintenance - Parts and fittings that are awkward and difficult to clean will add considerably to labor costs.
4. Durability - Fragile parts such as sight glasses should have adequate protection. Seals and breakable joints that have to be dismantled should be of good quality. Stopcocks, taps and valves should be corrosion proof.
Contamination problems may easily arise because of the nature of the substances involved in the processing from chemical action of one substance on another. E.g., heavy metals leached from the equipment are in themselves toxic contaminants, which may then trigger a further reaction in the substance being operated on.
Great care should be taken in the selection of materials that will be in contact with solvents or herb extracts. If using plastics or rubbers, then ask the supplier for the specifications of use. Do not use glaze ware unless you know what type of glaze it is. There are also several physical factors that need to be considered, e.g.,
A. Strength and Weight - Will the equipment be fixed or portable? Will the equipment be able to withstand any stresses placed upon it?
B. It�s Durability - Parts that are in contact with liquids and vapors must be resistant to corrosion. Metals that are prone to rust should as far as possible be avoided
C. Thermal Expansion and Conductivity - When mating materials, which are different, remember that they will have differing thermal expansion rates. That will produce stress or fatigue with an increased risk of fracture. Distillation equipment and condensers should possess good thermal conductivity.
D. Cleansing and Sterilizing - Smooth polished surfaces will simplify cleaning and sterilizing and help in the prevention of the formation of heat resistant films.
Two of the most commonly used materials for plant construction are copper and stainless steel. If considering copper, then it is most important that all linings in contact with the herbal materials, liquids or vapors, must be tin plated. Copper is a heavy metal that can cause liver damage, a known hepatoxic. Stainless steel will meet all criteria. Costs may be kept to a minimum by purchasing and modifying used vats, fittings and tubing.
The Heavy Metals 6.4
The root cause of many of the degenerative
diseases to which we are prone, may be found in the realm of the heavy
metals. In the normal course of events nature deals with them by using
naturally occurring Chelating agents, e.g., chlorophyll, hemoglobin and
citric, lactic, malic and tartaric acids. The
word �chelate� is taken from the Greek and means claw. The claw takes
hold of the offending atom of metal and aids its excretion from the body.
Without a chelating agent the heavy metal accumulates in the liver and other
organs in toxic amounts with predictable results.
Food and herbs grown using chemo-culture methods contains dangerous levels of heavy metals. Heavy metal contamination also occurs from contamination by processing equipment and storage containers; therefore caution is social responsibility which one may not neglect. The Monitoring and Assessment Research Center (MARC) Chelsea College. London, defines heavy metals as having an atomic weight higher than sodium and a specific gravity of more than 5.0 or over. That description fits more than 70 elements.
The American Environmental Protection Agency (EPA.) States that the most widespread of the heavy metals are as follows;
|
Arsenic |
Cadmium |
Chromium |
Copper |
|
Lead |
Mercury |
Nickel |
Zinc |
The Metals are listed alphabetically and not in
order of quantity as found in the environment.
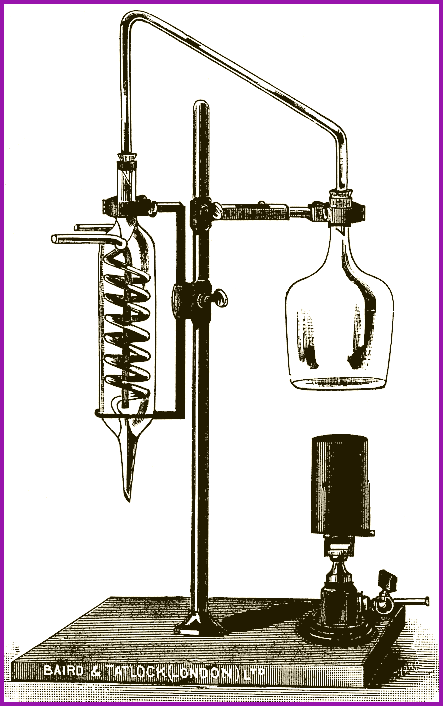
Distillation 6.5
The process of distillation involves a reversible
change of state i.e. Liquid --->
Vapour ---> Liquid.
A liquid is subjected to heat input to produce a vapour. The vapour is rapidly cooled to produce a liquid. Standard laboratory equipment is called a distillation train.
Simple Distillation Train Figure 6.5A
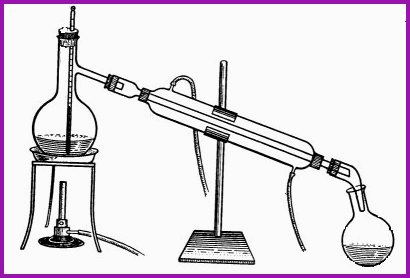
On the left is the boiling flask in which a liquid is steadily turned to vapour. Central is the water cooled condenser to change the vapour back to a liquid. On the right is the collecting vessel. Figure 6.5A represents the process in its basic form; to which one may add a thermometer to monitor the temperature of the liquid in the boiling flask. A heat source is needed to produce a sufficient volume of vapor from a liquid, and a cooling surface (condenser), to convert the vapor back to a liquid.
The Heat Source 6.6
If the liquid or vapor that is being
operated on is explosive or inflammable then special precautions must be
taken. If the liquid or vapor cannot be isolated, then an open flame as heat
source should be avoided. For small scale operations using an electric hot
plate or heating jacket is better, rather than a naked flame. If a naked
flame is used with glassware, then placing a square of metal gauze or
asbestos between the flame and the glass is advisable. This distributes the
heat and guards against thermal shock. Better still, the boiling flask
should stand in a water bath when distilling alcohol.
Temperature Regulation 6.7
Electric heating devices usually
incorporate a thermostat to regulate temperature with precision. Temperature
regulation is necessary to prevent decomposition of the product toward the
end of a distillation process, or to avoid damage to heat sensitive natural
products. Without a thermostat or thermometer an adequate control of
temperature may be gained by using water, sand or oil baths.
The water bath is also commonly known as the bain-marie and is extremely useful. It will give a fixed maximum temperature of 95�C, thereby preventing the charring of products contained within a flask. The water bath is also used extensively for the evaporation or concentration of liquids.
A Simple Water Bath. Figure 6.7A
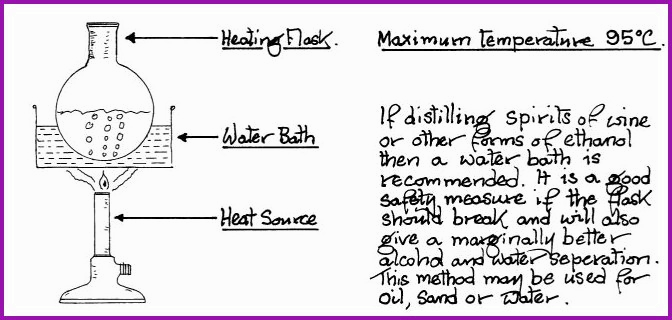
Evaporating Water Bath 6.7B
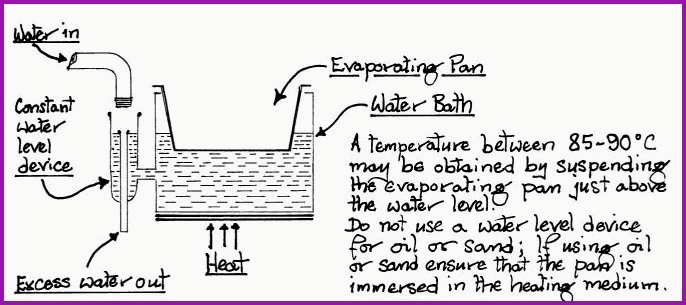
From the points covered by dehydration, it will be understood that all temperatures exceeding 60�C, herbal constituents are thermolabile and prone to damage. By substituting sand for water a fixed temperature of 105E C may be obtained. Substances such as glycerine and oil will produce temperatures between 150 and 300E C. However the Herbologist has no need to work at such temperatures, unless higher are needed for calcinations. Suffice to say that substances that decompose and release toxic vapours should be avoided. The points made in paragraph 6.6 should be noted.
The Condenser 6.8
An inadequate or inappropriate condenser
is the prime cause of failure in self built distillation equipment. The task
of the condenser is to rapidly lower the temperature of hot vapor to, or
below its dew point temperature, to precipitate a liquid.There are many
types of condensers, however, they all employ either air or a liquid as the
means of removing heat from a hot vapor. The water-cooled condenser meets
all the requirements.
When in use a condenser should be mounted in an oblique or vertical position to facilitate the drainage of the condensed vapors. The most common form of condenser is the tube within a tube, which on a laboratory scale is represented by the Liebig condenser as shown in Figure 6.5A. Alternatively, for greater efficiency in larger scale operations, the condenser may be multi tube. If the amount of cooling water available is a consideration then a coil within a tank will meet the requirements. The coil within the tank is usually referred to as a �worm�.
Figure 6.8A. The Multi tube and Coil type Condensers.
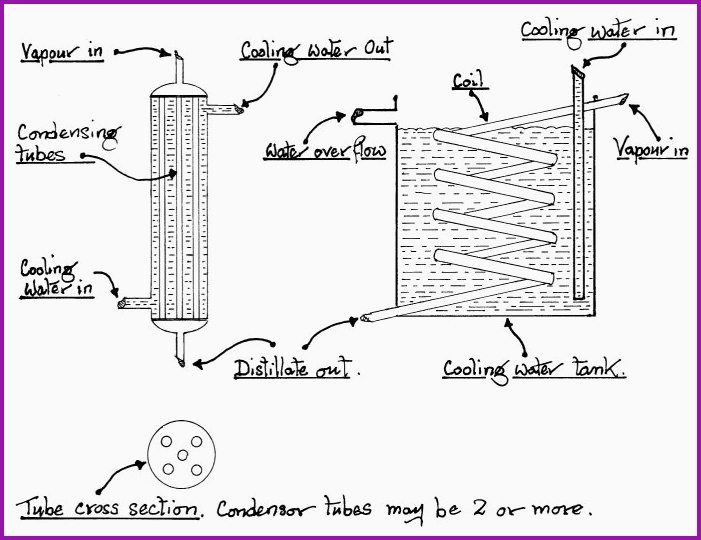
If using a condenser of the coil in the tank type, it will be found that the upper layer of water will heat up quite rapidly; this will effect the efficiency of the condenser, and it should be removed via the overflow pipe, by introducing fresh cooling water to the tank.
The heat energy may be partially recovered by feeding the warm overflow water back to the body of the still. If the still is operating continuously, then considerable economy of energy may be achieved by having a continuous regulated flow of cooling water to the tank. See Figure 6.19A. To calculate the rate of flow, proceed as follows;
ExampleCooling water into the tank = 10 C
Temperature of the distillate = 40 C
Temperature of the steam = 100 C
Obviously the temperature of the distillate may vary considerably; however, in this example the temperature difference between the steam and the distillate is 60�C. The following values are taken from Figure 4.38A.
1 kg of steam, on condensing, will release 2260 kJ of heat plus 4.2 kJ/kg/�C, i.e., 60 x 4.2 = 252 kJ, giving a total heat surrender of 2512 kJ/kg.
For reasons that will be explained later, the water that is fed back to the still should ideally be at a temperature of 90�C. Therefore, if the cooling water temperature is 10�C, we have 90�C - 10�C = 80�C.
To raise the temperature of the cooling water it
will need 4.2 kJ/kg/�C, i.e., 336 kJ/kg. Theoretically, 1 kg
of condensed steam will heat,
Steam/kJ/kg 2512 � 336 = 7.4kg of feed
water.
for each kg of distillate produced. If the
still output is 10 kg (litre) per hour, then 74 litres of cooling water must
be added in one hour, or 810 ml per minute. In practice, due to unavoidable
losses, this figure may be reduced by 20%. To
achieve the correct flow, simply adjust the cooling water tap until the
still feed water is around 90�C. The temperature should be taken at regular
intervals, and cooling water adjusted accordingly.
Condenser Efficiency 6.9
The heat transfer rate of a condenser must
exceed the enthalpy of vaporization produced by the still, i.e., it must be
capable of condensing the vapor produced. The amount of vapor generated is
dependent on heat input per unit of time. Therefore,
if purchasing a commercial unit, they will be designed to produce a given
amount of distillate, in a given amount of time, e.g., 10 litres per hour. A
condenser for such a still would need to cope with approximately 25,000
kJ/hr to convert all the vapor output from the still. For
practical reasons, the condenser capacity must exceed the theoretical heat
load by some 20%. The efficiency of the condenser will depend on the
following factors;
A. The area of its cooling surface.
B. Its thermal conductivity. The actual rate of heat transfer for a tube(s) within a tube condenser will also depend on the velocity of the cooling water across the condenser tube(s).
C. The extent of the vapor/cooling surface contact, which will depend on the diameter of the vapor tube and the velocity of the vapor.
D. The temperature difference between the vapor and the cooling water. These factors taken as a body, determine the capacity of the condenser.
Condenser Capacity 6.10
Steam produced within a still at
atmospheric pressure is said to be saturated, and can be seen as a mist or
fog, which is comprised, vapor and very finely divided water particles. If
it loses heat without a corresponding drop in pressure, the steam will
promptly condense to water. The following extract from the steam Tables will
help in making necessary calculations for the altitude at which a still may
be operated. Round the figures to the nearest whole number.
Steam Data.
Table
6.10A
|
Absolute Pressure (kPa) |
Saturation |
Sensible |
Enthalpy |
Total Heat |
Steam |
|
95.00 |
98.20 |
411.43 |
2261.80 |
2673.20 |
1.777 |
|
100.00 |
99.63 |
417.46 |
2258.00 |
2675.00 |
1.694 |
|
101.30* |
100.00 |
419.04 |
2257.00 |
2676.00 |
1.673 |
|
106.30 |
101.40 |
424.90 |
2253.30 |
2678.20 |
1.601 |
* Standard atmosphere. Sea level.
For example, let us assume that we have a single tube stainless steel condenser, and we want to know whether it will be suitable for a still, with an output of 10 litres of distilled water per hour.
Step 1. Calculate the area of the condensing tube. Example the tube is 0.750m long and a diameter of 1 cm
Area = Circumference of the tube x length
D x L = 3.14 x 1 x 750 = 2355cm = 0.235m�
Step 2. Consult Table 5.25A and determine the thermal conductivity, i.e., stainless steel 20 J/second/m� Remember that 1 watt = 1 Joule per second.
Step 3. Determine the difference between the saturated vapor temperature and that of the distillate. Assume distillate temperature as 40�C. therefore 100 � 40 = 60�C difference
Step 4. Calculate the thermal conductivity of the condenser tube, the thickness of the condenser tube wall is 1 mm.
Area x Conductivity x Temperature � Wall thickness. 0.235 x 20 x 60 � 0.01 = 28.20 kJ/second.
A still with a capacity of 10 litres/hr produces 1 litre every 6 minutes, or 1 kg of saturated steam every 6 minutes. Table 6 -10A shows that 1 kg of steam at atmospheric pressure, holds 2676 kJ of heat energy. Therefore at 28.2 kJ/sec the condenser tube can disperse 10,152 kJ in 6 minutes.
Thermal Conductivity of a Spiral Coil
6.11
Step 1. Determine the length of the tube
in the coil by measuring the outer and inner diameter of 1 coil, add them
and divide by 2, this will give the average diameter.
Step 2. Multiply the average diameter by the p (3.14), this will average circumference.
Step 3. Multiply the circumference by the number of coils, which gives an approximate length of the tube in the spiral.
Step 4. Proceed as in section 6 -10.
N.B. Due to simplification, the calculation method is not strictly accurate. However the margin of error is very small and will meet our purpose.
Distillation Techniques and Products
6.12
Distillation is important to the
Herbologist in three main areas; Concentration � Purification �
Separation. To achieve those ends four
techniques are employed;
1. Simple distillation which is as Paragraph 6.5.
2. Fractional distillation of a substance, where components of different boiling points are separated.
3. Distillation under reduced pressure to avoid damage or chemical change to a substance, where a change in physical form is required.
4. Distillation in steam. This is the method most often used for the production of volatile oils.
There is one further technique, which is destructive distillation or pyrolysis, which will be covered under the heading of sublimation. For the Herbologist the two major products of distillation are the solvents, i.e., purified water and ethanol, without which, the science would be no more than a primitive art. From the techniques a diverse range of products and by products are obtained. For example;
Purified or distilled water � Aromatic waters � Ethanol � Essences/Spirits
Volatile oils � Extracts � Tinctures � Benzoic acid.
Boiling Points 6.13
Boiling, or ebullition, is the process
that constitutes the difference between evaporation and distillation. When
the vapor pressure of a liquid exceeds the pressure upon it, the liquid will
commence to boil, (see Figure 4.44A). The boiling point of a pure substance
is clearly defined and will boil at a given temperature for a given
pressure. Therefore, a fluctuation in pressure will alter the boiling point.
Consequently if using boiling points for identifying a substance, then
pressure is a prime concern.
The organic compounds are organized into families, which have a similar shaped molecule and are called �homologous series�. For example the alcohol family comprises more than 70 members. Generally, the boiling points of a series will rise in line with an increase in molecular weight. Water does not belong to a homologous series.
Representative Alcohols. Table 6.13A
|
Alcohol |
Molecular weight |
Boiling point |
|
Methanol |
32.0 |
64.65�C |
|
Ethanol |
46.1 |
78.52�C |
|
Amyl (fusel) |
88.1 |
138.25�C |
|
Glycerol |
92.1 |
290.0�C |
Ethanol as a pure substance cannot be separated completely from water by the distillation process. This is because, when ethanol is at a concentration of 95.5% it forms a constant boiling mixture with the 4.5% water, which will distill over with it. The subject of ethanol will be covered in greater detail in the module that deal with solvents used and their preparation.
Vapour Composition Table 6.12B
|
Temperature �C |
% Ethanol |
% Water |
|
78.5 |
86 |
14 |
|
80.0 |
83 |
17 |
|
82.0 |
79 |
21 |
|
84.0 |
76 |
24 |
|
86.0 |
72 |
28 |
|
88.0 |
67 |
33 |
|
90.0 |
62 |
38 |
Library
Pharmageddon
Herbal Block Index
![]()