
|
|
|
-::-::- Introduction The beautiful symbolism of the image on the left is called �Return of Persephone� it was painted by Lord Frederick Leighton. 1830-1896. The original is held at the City Art Gallery, Leeds, UK. It depicts the return of Persephone to her Mother, Demeter the Greek Earth Goddess. Demeter had been kidnapped by Hades the King of the Underworld. Demeter caused famines and sickness, whilst in mourning for her beloved daughter. Zeus
the King of the Gods was
prevailed upon to send Hermes to return Persephone to her
Mother. It is a splendid allegory of our age. |
The old Hermetic belief of hierarchies of order are reflected in the Christian and Muslim religions in the form of Saints, Angels and Demons, no matter whether Buddhist, Hindu or Taoist, the terminology may differ, but the story is the same. This is self evident, no matter whether we see through Telescopes, or Microscopes. That magnificent order may be seen from the smallest to the largest. From Galaxy to the Cell in an organism. Just as Newlands Chemical table of Octaves was the foundation stone of the modern Table of the Elements. Then we may see that the same sort of Octave relationship, between cell and galaxy, this in order, of scale of magnitude.
This understanding, is a core concept of Vitalism. The Tree of Life, the universal symbol of human understanding. This knowledge is as ancient as our kind, and is rooted in Shamanism. This knowing ledge or overview, of being part of a far greater organism, gives to life, a reverence and sanctity, which is sadly lacking in mainstream medicine and food production. That lack of reverence is also seen in those arms of science, commerce and politics that give support to, and mould that desecration. The evidence of which is all around us.
-::-::-
Preparation
of Fermentation Liquids 9.16
The efficiency of the
sugar fungi, relating to alcohol production, is determined by their
environment and the substrata on which they feed. From
Table 9.11A,
it will be seen that the carbohydrates (CHO) are divided into three
divisions.
|
1. Monosaccharides |
Direct Fermentation. |
|
2. Disaccharides |
Inversion + Fermentation. |
|
3. Polysaccharides |
Hydrolysis + Fermentation |
Some carbohydrates, i.e., �Poly and Di�, must be converted to mono form for fermentation to commence. That involves an expenditure of energy. Starch cannot be fermented directly, it must first be broken down to Disaccharide, then monosaccharide form.
This may be done with hydrochloric acid (not recommended), or by a malting process (see Figure 9.11B), or by the addition of the �Amylase�. The probable pathway is as Figure 9.11B. Amylase converts starches into sugars, to power a plants metabolic process.
Amylase is readily available as a commercial product. We cannot make it, we borrow it from plant sources. The starch grains and tubers, such as wheat, barley, potatoes, etc., can, under the right conditions, be made to sprout. This is the first step from dormancy to the growth of a new plant.
The amylase is triggered and commences to convert the stored starch into sugars. If the process is checked, and the seed or tuber killed, the sugars can then be utilized by the sugar fungi. The process is simplified if the plant material used, already contains useable sugars, e.g., grapes, apples, etc., in such cases it is usually sufficient to crush or pulverize the material, and then ferment the juices.
 If the yeast substrata is a disaccharide, such as cane sugar, it may be fermented directly,
but as may be seen from Figure 9.12B, the yeast must first turn
the disaccharide sucrose into the monosaccharide forms, i.e., glucose and
fructose by secreting sucrose. If the yeast substrata is a disaccharide, such as cane sugar, it may be fermented directly,
but as may be seen from Figure 9.12B, the yeast must first turn
the disaccharide sucrose into the monosaccharide forms, i.e., glucose and
fructose by secreting sucrose.
We can speed the process up, by inverting the sugar solution. by boiling it and taking care the liquid is reduced in temperature, before adding the yeast. Temperatures exceeding 35�C are to be avoided if we do not wish to kill the yeast. Malt or molasses may be fermented directly, if first diluted with water. The carbohydrates are found as complexes in all higher plants, those that occur in the lower plants, such as the ferns, mosses and their allies, are substantially the same, therefore, with the correct treatment, we may use them as fermentation substrata, with or without, the addition of sucrose. Remember that carbohydrate synthesis in plants is photosynthesis. The following Table may be used as a rule of thumb to estimate the amount of plant material required, to produce a given amount of alcohol.
|
| Carbohydrate | Fresh Weight | Dry Weight |
| Root - Beet | 9.4% | 76.4% |
| Tuber - Potato | 16.5% | 67.5% |
| Leaf - Spinach | 2.9% | 50.8% |
| Seed - Wheat | 70.4% | 81.5% |
The rule is; that all such plant parts must be treated with amylase to convert the carbohydrate to fermentable sugars. We may see from the above table, that the leaf part of the plant contains the least amount of carbohydrate. In order to reduce the bulk of leaf material used, when a large quality of alcohol is required, it is permissible to add sugar during the fermentation process to increase the alcohol yield,. If however, a fermentation is part of a Spagyric process, sugar should not be added, the reasons will be discussed when Spagyric preparations are covered.
Factors that Influence Fermentation 9.17
The carbohydrates
are broken down by many different types of micro-organisms, each one of which,
produces its own end product. The different tribes of the sugar fungi produce
different types of alcohol, e.g. lactic fermentation will produce amyl alcohol,
which is one of the fusel variety, and therefore, not required.
Accordingly, the must or fermentation liquid should be sterilized before inoculation with wine yeast, in that way, it does not have to compete with other fungi for resources. The most common method of sterilizing a must, is by the use of sodium metabisulphite, which at the correct concentration, will kill unwanted micro-organisms, if however, the concentration is too high, it will inhibit or kill the wine yeast. This procedure is not recommended.
By far the best method of sterilization, is to raise the temperature of a must to 60�C and hold it there for 45 minutes. During the heating and cooling period, the must should be kept covered to avoid re-contamination. When the must is inoculated with the wine yeast, the fermentation must be carried out anaerobically, i.e., without the presence of oxygen, which if present, will allow other yeast or bacteria which convert sugars to vinegar, to outstrip the growth of the wine yeast. Exclusion of oxygen is achieved by use of an air lock.
If the sugar content of a must is too high, that will also immobilize the yeast, and like incorrect temperature, is one of the most common causes of a stuck ferment. The preservative powers of sugar in high concentrations is utilized in many food products, e.g. jams and preserves, where the high concentrations of sugar inhibits the growth of the micro-organisms.
Pure grape juice can contain up to 25% of fermentable sugars, therefore, we should take that hint from nature and ensure that a starting must contains no more than that amount. Refer to Table 9.14B - column 2, which is the Twaddell scale, and we will see that 25% sugar in 5 litre, has SG of 1125, which should not be exceeded.
To achieve a good sugar to alcohol conversion ratio, it is best to start with a lower SG and dose the yeast with invert sugar at regular intervals, in that way a vigorous fermentation in maintained.
Ethanol is a waste product of yeast enzyme activity; and as its concentration in the fermentation liquid starts to rise, the integrity of the yeast colony is adversely affected by its own excreta and activity declines until the yeast is immobilized.
Nature has given us a lesson in ecology. At what concentration that immobilization occurs, is in many respects, dependent on factors over which we have no control, we can only provide an estimate of optimum conditions, the rest is up to the yeast. Concentrations around 18% by volume have been achieved in strictly controlled laboratory work, but in practice, it is rare to achieve more than 15%, with 12 to 13% being the norm before fermentation ceases.
The Hydrometer and Fermentation Liquids 9.18
By comparing
a hydrometer reading taken in a fermentation liquid, with
Table 9.14B,
we can obtain five essential
pieces of information.
1. How much sugar is present (refer to Section 9.15).
2. Based on sugar content, an estimate of
potential alcohol by volume.
3. How much sugar is required to produce a
desired level of alcohol.
4. An indication of fermentation progress.
5. An approximation of alcohol present, when
the fermentation has ceased.
Ideally, the SG of a starting liquid should be between SG 1.060 and 1.070. The following figures are taken from Table 9.14B, and are based on a 5 litre batch of liquid. On the assumption that the temperature of the starting liquid is 20�C, we proceed as follows.
| 1. Estimating how much sugar is present. | |||||
| SG reading 1.034 (column 1) | |||||
| Deduct 0.007 (contains non fermentable substances) | |||||
| Add 0.003 (temperature / buoyancy Table 10-15B) | |||||
| Actual SG 1.030 (sugar present = column 5) | |||||
| 2. SG 1.030 = potential alcohol by volume 4% (column 3). The gravity is 30 (column 6). | |||||
| 3. Required alcohol by volume 14% (column 3) The gravity is 110 (column 6). | |||||
|
� |
Required alcohol = Gravity 110 | ||||
| SG of liquid = Gravity 30 | |||||
| Difference = Gravity 80 | |||||
| Gravity 80 (column 6) = 1272 grams of sugar (column 5), to be added to produce a liquid of 14% alcohol by volume. We may considerably simplify calculations by referring the SG reading to gravity, in column 6. | |||||
| 4. The measure of the work done by the yeast on the sugar content of the liquid, may be measured by SG or gravity drop. From Table 9.14A, we may see that the SG of sugar is higher than water, and the SG of alcohol is lower, therefore, as the yeast goes to work on the sugar by converting it to alcohol, the gravity will drop accordingly. For example; | |||||
| SG of starting liquid 1.065 = Gravity 65 | |||||
| SG of a random check 1.055 = Gravity 55 | |||||
| The difference is Gravity 10 | |||||
| Using the Table 9.14B, we may see that a gravity of 10, indicates that 159 grams of sugar has been converted, and proceeding to column 3, we can see that the liquid now contains around 1.4% alcohol by volume. | |||||
| Gravity 65 = 1033 grams of sugar. | |||||
| Gravity 10 = 159 grams of sugar | |||||
| The difference = 874 grams of sugar remaining. | |||||
| 5. When a fermentation has ceased, take a final SG reading, and deduct that reading the from the starting SG. The gravity difference represents the approximate alcohol by volume. | |||||
| From those readings, we may also deduce the efficiency of the yeast colony, by the amount of un-fermented sugar left, e.g | |||||
| Starting SG after adjustments, 1.115 = Gravity 115 | |||||
| Final SG after fermentation 1.015 = Gravity 15 | |||||
| Difference between the two = Gravity 100 | |||||
| Read off the gravity values from Table 9.14A. | |||||
| Gravity 115 | = | Potential starting alcohol (column 3) | = | 14.9% | |
| Gravity 15 | = | Final gravity - sugar left (column 5) | = | 238g sugar left. | |
| Gravity 100 | = | Gravity drop, potential alcohol (column 3). | = | 13% alcohol. | |
| When commencing a fermentation process, an adequate record should be kept of SG readings, so that correct calculations may be made. | |||||
| The Preparation and Use of Invert Sugar 9.19 | |||||
| For our purpose, invert sugar with a minor difference, is essentially the syrup of the British Pharmacopoeia 1958. The formula is as follows; | |||||
|
Refined
Granulated Sugar - 667 g (Sucrose) Distilled Water to Produce - 1000g. Its weight at 20�C is 1.31 to 1.33 g/ml. Its SG at 20�C is 1.310 to 1.334 |
|||||

It will be recalled that sucrose is a disaccharide, therefore, we must invert the syrup to a monosaccharide form, i.e. sucrose into glucose and fructose, this done by adding acid to the syrup. For that purpose, we use Citric acid, either in a pure crystallized form, or as fresh lemon juice. The amount required is small, and approximates 2 g citric acid per kg of sugar, or 0.2% by weight. That small amount is sufficient to bring about a decomposition reaction in 500 times its own weight. The student will do well to remember this, when considering glycerine, acetic acid or vinegar as a solvent or carrier. The British Pharmacopoeia formula, requires 667 g of sugar. To calculate the amount of citric acid required proceed as follows; |
Sugar 667g - Acid required is 0.2% by weight
� 667 x 2 = 1334 mg or 1.5 g.
It is essential that the quantities specified are adhered to, because too little sugar will result in changes due to spoilage organisms, and too much will result in the inverted sugar crystallizing out in a form that severely affects its solubility. The citric acid will cause a darkening of the syrup on standing. The acidified syrup should be retained purely for fermentation purposes. Correctly prepared, it will keep indefinitely when sealed to exclude moisture and micro-organisms.
To prepare the syrup, place all the ingredients in a suitable container and mix. Bring the solution to 80�C and hold it at the temperature for 20 minutes with constant stirring. Do not allow the syrup to boil, and skim any scum that may rise. When the syrup has cooled it is ready for use, or it may be poured into suitable containers and stored.
1 ml syrup contains 0.667 g sugar. 1 litre contains 667 g sugar.
Syruping the Fermentation Liquid 9.20
Syruping is the term
used for dosing the fermentation liquid with syrup at appropriate intervals. If
the procedure is carried out correctly, then high levels of alcohol may be achieved consistently,
eliminating the hit or miss of the un-managed fermentation. Refer back to
Table 9.14B, and assume that after the
appropriate adjustments for temperature and other substances in the must, that
its SG is 1.030, or Gravity 30 (column 6), and that we require a liquid that
contains 14% alcohol by volume, i.e., Gravity 110,
|
|
Required alcohol - Gravity 110 - Target level. Potential alcohol - Gravity 30 - Actual level. The difference - Gravity 80 = 1272 g sugar. |
||
|
1 ml syrup contains
0.667 g sugar |
|||
| � |
Sugar required 1272 g � 0.667 = 1907 ml of syrup. |
||
| The syrup is then divided into doses. A good rule of thumb is; | |||
| Syrup required - 1
litre or under �
2. Syrup required - 2 litre to 1 litre � 3. |
|||
Therefore, we require 1907 ml syrup to reach the target level = 635 ml in 3 doses.
When to Syrup 9.21
Ideally, a new fermentation liquid
should not exceed an SG 1.070
(Gravity 70), in this way, we
can be sure that the yeast will
be stimulated and not inhibited by an excess of sugar. When the
yeast culture is introduced to the liquid, the fermentation process has three
distinct phases, we will label them Primary, Secondary and Tertiary.
The first stage in the primary phase is known as the lag stage, during which, a barely detectable fermentation occurs. The yeast colony metabolizes just sufficient sugar for it to multiply into the available food source. When the colony reaches optimum size, the primary phase proper commences.
The fermentation is extremely vigorous to the point of frothing. The carbon dioxide released is visible in the form of bubbles which burst with an audible hiss. The phase can vary between 2 and 7 days, depending on temperature and substrata.
The SG of the liquid should be taken daily. It will be noticed during the primary phase that the gravity drop is rapid. The gravity should not be allowed to drop below 20. Between gravity 25 and 20, introduce the first dose of syrup, ensuring that it is well dispersed by vigorous stirring.
When the gravity again drops to 25, add the next dose of syrup and disperse by stirring. That procedure is repeated until the specified doses of syrup have been used. During the syruping procedure the primary fermentation will slow as the ferment enters the secondary phase.
Secondary and Tertiary Fermentation 9.22
The secondary phase
is characterized by a steady fermentation, which is visible and audible, with
carbon dioxide being produced at a steady rate. The gravity drop is less
dramatic than that of the primary phase, and will last for around 7 to 10 days.
At approximately gravity 15, the fermentation enters the tertiary and final phase. The sugars are being steadily consumed and the alcohol level will be around 12 to 13%. The activity of the yeast colony declines rapidly towards dormancy. If subsequent SG readings, across a 24 to 36 hour period, show no change and indicate low sugar levels, then it may be assumed that the fermentation is complete.
The hydro - alcoholic liquid may then be prepared for the distillation process. This is quite simply a process of siphoning off and filtering. The residue left at the bottom of the fermentation vessel is known as �Lee,s� which amongst other substances contains the bulk of the dormant yeast colony, which may be used as the starting nucleus for a fresh fermentation. It requires an alcohol level around 70%, to kill the yeast.
Culturing the Yeast 9.23
Yeast for fermentation
may be obtained from retail or wholesale outlets that cater for the amateur
wine-making fraternity. The yeasts are available in great variety, both in dried
and liquid form.
As previously stated, we have no interest in bouquet or nuance of flavor, we seek maximum conversion of sugar to alcohol. For that purpose, a red wine variety, such as a Burgundy or Tokay yeast, which are consistent performers in the top end of alcohol production.
When purchased, the yeast will be dormant and will need to be activated for inoculation of the fermentation liquid. For activation, a glass bottle and a wad of cotton wool to seal the neck may be used as the culture chamber. The bottle should be clean and well rinsed.
The activation takes place in a sugar containing liquid at the optimum pH (see paragraph 9.8) The liquid is usually taken from the prepared must. Alternatively, it may be prepared from sugar, water, lemon juice and nutrients. Nutrient salts may be purchased with the yeast.
Good results may be obtained with half of a crumbled multi-vitamin tablet that contains Thiamine (B1). The ingredients are mixed by swirling in the bottle. The yeast is added and the bottle closed with the cotton wool.
The temperature should be held between 20 and 25�C. Within 2 to 3 hours, the yeast culture should be frothing. At that point it may be added to the must, and well stirred in. The fermentation vessel is then sealed so that an anaerobic fermentation ensues.
The Fermentation Airlock 9.24
The airlock
is essentially a plumbers �U� bend,
|
It is a water trap, that allows the escape of carbon dioxide, but denies the access of free oxygen or micro-organisms to the fermenting liquid. The fermentation process produces carbon dioxide, which occupies the air space above the liquid. As the pressure increases from its production, it forces its way past the water in the bend of the airlock. The water prevents the access of air or contaminants from the atmosphere The airlock is an essential piece of equipment for anaerobic fermentation inside a closed brewing container. The evolution of the CO2 maintains a slightly higher pressure inside the container than that of the atmospheric air. As the Carbon dioxide is produced, it will force itself past the water barrier in the lock and escape to the atmospheric air. During the secondary fermentation phase, the CO2 will escape in a steady stream of bubbles which are quite audible. |
Sterilisation 9.25
The equipment used for the
fermentation process must be kept scrupulously clean. This is easily and
economically achieved by washing equipment in a solution of sodium metabisulphite,
which is used in concentrations
between 100 and 150 ppm.
The packet will contain precise instructions for preparing different strength
solutions. If it is to be used to sterilize a must, then great care must be taken lest the yeast be killed
or unduly inhibited. The water
used in the airlock should be sulfated to prevent micro-organisms growing in it.
Summary of the Fermentation Process 9.26
 The fermentation substrata is selected from the Plant Kingdom. The material may be classed as monosaccharide, disaccharide or polysaccharide. The structure of the carbohydrate will determine the process route, i.e., from substrata to alcohol. Remember that the yeast is a living organism, and that we only manage the process to achieve optimum results. This involves the provision of nutrients, the adjustment of pH, and the correct temperature. The fermented liquid is a
complex chemical soup
and that sugars and
alcohol are only two substances amongst many. Accordingly, precise
measurement of alcohol content is not feasible. |
The alcohol content can only be estimated if we keep a careful log of all SG readings and the subsequent gravity drops. If syruping is done, then each gravity rise and fall is recorded. At the end of the process, the total gravity drops are added together, and the total is read off from Table 9.14B. This is the estimated alcohol, e.g.
| The starting SG. | 1.060 | Gravity drops to 1.025 | Gravity 35 | |
| SG of 1st Syruping. | 1.065 | Gravity drops to 1.025 | Gravity 40 | |
| SG of 2nd Syruping. | 1.065 | Gravity drops to 1.025 | Gravity 35 | |
| Final SG reading. | 1.025 | Total Gravity Drop | Gravity 110 |
From Table 9.14B, go to column 6, and locate gravity 110, then go across to the Baum� Scale, column 3, and read off the estimated alcohol content, i.e. 14.2% alcohol.
Production Routes. Table 9.26A
|
Substrata |
Monosaccharide |
Disaccharide |
Polysaccharide |
|
Type |
Fruit, Pip or Stone |
Sugar |
Tuber-Leaf-Grain |
|
1st Treatment |
Pulp or Crush |
Hot Water |
Crush-Chop-Grind |
|
2nd Treatment |
Hot Water |
Invert |
Hot Water |
|
3rd Treatment |
Ferment |
Ferment |
Add Amylase |
|
4th Treatment |
- |
- |
Ferment |
|
5th Treatment |
Siphon - Filter |
Siphon - Filter |
Siphon - Filter |
NB. Honey, Malt or Molasses may be treated as a monosaccharide. Commence the process at the 2nd treatment.
Amylase, like yeast is heat sensitive, therefore, a polysaccharide liquid should be allowed to cool to 25�C before adding the amylase. To test if the starch has been converted to sugars, half fill a test tube with the liquid and add 1 or 2 drops of tincture of iodine. If the starch is present it will be indicated by a purple/black color change. If a significant change is noted, leave the ferment liquid for a few hours longer to allow the amylase to complete its task. If the color change is persistent, add more amylase.
Prior to fermentation, ensure that the following adjustments are made.
1. Adjust the volume of
the liquid to the volume required for the complete ferment.
2. Adjust the SG. To lower the SG
add water; to raise the SG
add more substrata or syrup.
3.
Adjust the pH.
4. Adjust the
temperature
to between 20� to 25�C.
Fermentation Products 9.27
The fungi and bacteria are used in
great variety in the food and pharmaceutical industry.
It is beyond the scope of this text to even briefly cover the species and
technology employed. However, the student is encouraged to study the matter for
themselves.
The conversion of natural substrata by microbes into proteins and vitamins, is a legitimate sphere of activity for the Herbologist. Indeed Nutrient and Medicinal products are produced in great variety by fermenting an appropriate substrata with CANDIDA UTILIS, which is the source of our food yeasts. Elementary text books on microbiology will yield the information required for production and the cultural mediums required.
Maintaining a Yeast Strain 9.28
It is advisable
to purchase an initial yeast colony of a well proven strain. Thereafter, with
the proviso that the same type substrata is used for fermentation, the original
colony will adapt its offspring to match the conditions given. Such adaptations
will perform consistently.
After fermentation, filter around 500 ml of the remaining lees, through a standard filter paper. Fold the paper and roll it into a pencil shape. Place the paper in a large test tube. Add sufficient 16% by volume Glycerine solution, to immerse the paper. Seal the test tube. Store in your deep freeze until required.
To reactivate the culture allow to thaw, unfold the filter paper and allow the glycerine solution to drain, and float it in the fermentation liquid where it will eventually sink. The reactivated colony will proceed as normal. When the fermented liquid has been siphoned off the lees, then culture a further colony as previously described.
Distillation of Alcohol 9.29
The technicalities
and the theoretical points surrounding distillation, are covered in
Module
6,
therefore, you may find it helpful to
refresh your memory before proceeding.
The alcohols are representative of a homologous series. Their molecular weights and boiling points display the usual gradation observed in other series. The alcohols are classed as Primary, these are liquids which are miscible with water. Secondary alcohols are also liquids, but are not completely miscible with water; tertiary alcohols are greasy solids.
Our interest at this point, lies with the primary alcohols (see Table 6.13A). As a general rule, the primary alcohols form an azeotropic (constant boiling) mixture with water, i.e., they behave as though they are pure liquid. A two liquid azeotrope, such as alcohol and water, are called binary azeotropes, and will boil off at a constant temperature until the substance with the lower boiling point has been separated.
As the separation becomes complete, the temperature rises to the next highest boiling point, and that substance commences to boil off. This may be more clearly understood by the following distillation curve for a binary azeotrope of alcohol and water. The mixture is 65% alcohol by volume.
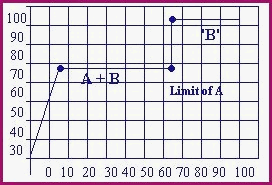
The row of figures at the bottom of the chart are % by volume. B.P. Alcohol 78.5�C
(A) The curve is not to scale and is for illustrative purposes only. |
Assume a 5 litre fermented soup that contains 15% ethanol by volume, i.e. 750 ml of alcohol. The remaining 4.25 litre will be water and other substances, which will include amyl alcohol and possibly glycerol.
As an azeotrope, it is considerably more complex than a simple two compound one. Accordingly, it is not possible to predict with any great precision, the boiling point of such a mixture. However, experiences will teach you that the first running of alcohol from the condenser, commence when the temperature reaches about 82�C.
It is important to remember that a liquid does not have to be at boiling point to vaporize, and that water does so at all temperatures about 0�C.
Therefore, if resorting to simple distillation as the separation technique, the procedure will need to repeated a number of times to effect a high alcohol separation. Such a procedure is tedious in time and expensive in energy, therefore, it is better to use a simple fractionating column to achieve the separation of the alcohol in 1 or 2 runs, depending on the efficiency of the column.
Irrespective of the distillation method, the maximum level of alcohol produced cannot exceed 95.5% by volume. At that level, the alcohol and remaining water form a constant boiling compound and distill over together. Further separation can only be achieved by adding chemicals to bind the water and re-distilling. However, this is not required for the purpose of producing herbal medicines.
From the 95% alcohol we produce the purified or rectified spirit of the Pharmacopoeia, which is 90% alcohol freed from impurities and fusel oils; the diluting medium is double distilled water. It is permissible to use commercially available wines and spirits to produce the rectified alcohol.
If using brandy, rum, vodka, whiskey to produce 95% alcohol, then it is a matter of importance to ensure that the starting point of the alcohol was a fermentation process, and that the substrata used was one of those given in Figure 9.26A.
There are some very sound chemical reasons why this is necessary. For example, the substrata used to produce many of the cheaper spirits of commerce, especially gin and vodka, are the waste products of industrial processes. It is then chemically flavored and if required, colored.
For the more expensive products, produced by traditional methods, there are also problems, such as the presence of amyl alcohols and other substances produced by chemical reaction during the maturing process. These chemical substances will in turn react with the chemical constituents of the herbal material. All alcohol used for extractive purposes must first be rectified.
The Distillation and Rectification of Alcohol 9.30
The requirements for
distillation equipment are covered in Module 6. If using laboratory glassware,
do not forget to add some anti-bumping granules to the distilling flask .
Fractional distillation must be carried out with care. The distilling vessel
should be heated by a water bath or jacket, the steady gentle transfer of heat
from the water to the vessel will give an excellent separation.
Alcohol is a volatile inflammable liquid. Careless or sloppy procedures, may result in explosion or fire. All joints and seals on the apparatus must be airtight. The container for the collection of the distillate should be mounted away from the heat source. The operational procedure is as follows;
1. Introduce the fermented liquid to the
still.
2. Ensure all joints and seals are tight.
3. If using a tube in tube condenser, ensure
that the cooling water is turned on.
4. Turn on the heat source and bring the
liquid up to boil.
5. If there is an escape of vapor, other than
from the condenser switch of the heat and, rectify the problem.
6. When the liquid starts to boil, adjust the
heat input, so the run of distillate is steady with no escape of vapor.
7. When the distillation is
complete turn off the heat.
The apparatus will take a little time to reach operational temperature. If a glass fractionating column is used, then the slow rise of vapors can be observed ascending the column.
When the apparatus has reached operational temperature, about 80 to 82�C, and the operation commences, a constant eye should be kept on the thermometer. The temperature will remain, with very minor fluctuations, around the 80�C mark, for so as long as there is alcohol in the liquid undergoing distillation. When the last of the alcohol leaves the liquid, there will be a sharp rise in temperature up to the boiling point of water 100�C. At the first indication of that rise, the heat should be turned off, if undue contamination of the separated alcohol is to be avoided.
On the assumption of a 5 litre batch of liquid at 15% alcohol by volume, we would expect circa 700 to 750 ml of alcohol to be present in the distillate reservoir. Transfer that liquid to a clean sealed container. The apparatus should then be stripped down for a thorough cleaning. The liquid remaining in the still may be discarded.
If the fractional distillation has been carried out correctly, the distillate should be high strength alcohol, circa 95% by volume. Should that be the case, the alcohol is now ready for the rectification . The alcohol obtained is not pure, it will also contain the amyl alcohols (fusel oils). These must be removed from the alcoholic distillate, add sufficient distilled water (circa 15% by volume) to the alcohol.
Shake the mixture and allow to stand. The addition of water will precipitate the fusel oils. The mixture should then be double filtered through activated bone charcoal. The subject of filtration will be covered later in the text. When the filtration is complete, the liquid is again subjected to fractional distillation to separate the excess water.
When satisfied that the alcohol is at the correct strength, it is then filtered through activated vegetable charcoal to deodorize it. After filtration, it is then adjusted to 90% alcohol by volume with distilled water. It is now ready for use. Alcohol (Ethanol) that has not been rectified, should not be used for extracting plant material. To do so, introduces unwanted chemical reactions with the soluble constituents of the plant.
SG and Alcohol Strength 9.31
There are large margins of
uncertainty regarding the estimation of potential, or actual alcohol in a
fermentation liquid. This is because of the large number of chemical entities in
a such a soup, each one of which, when diluted in water, has its own relative density.
The distillate obtained from the first fractional treatment, and in comparison with the fermentation liquid, is relatively pure, being comprised in the main of alcohol, water and small fractions of amyl alcohols. Therefore, we may determine the alcohol strength of such liquids with a good degree of accuracy, which is further enhanced when the liquid has been rectified.
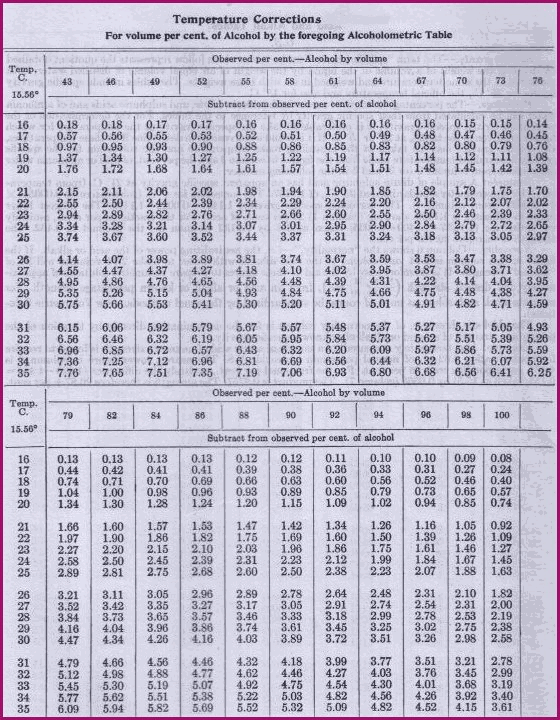
The relative density of the liquid is compared to that of double distilled water, and the difference is referred to the Alcometric Table. The method requires a 100ml measuring cylinder, a thermometer and scales sufficiently sensitive to weigh down to 0.1 g. The procedure is as follows;
|
1. Note the temperature of the atmosphere, and of the liquids to be weighed . It is important to ensure that the liquids involved are at the same temperature. They should be at the temperature to which the cylinder has been calibrated. 2. Weigh the clean dry cylinder and note the weight. 3. Accurately dispense and weigh 50 ml of the distilled water and note the weight. It is important that this is done, because if it weighs more than 50 g after temperature corrections have been made, then it is contaminated. 4. Empty the distilled water and thoroughly dry the cylinder, then accurately dispense and weigh 50 ml of the liquid to be compared, and note the weight. 5. Subtract the weight of the cylinder from each of the liquid weights. 6. Subtract the figure of 0.1 g from the alcoholic liquid to compensate for the weight/volume differences due to temperature. |
7. Divide the weight of the alcohol containing liquid by the weight of the distilled water.
8. Then read off the figure obtained against the Alcometric Table, to give the percentage of alcohol by volume.
For example, after the corrections to weight have been made,
we have the following figures;
50ml Alcoholic solution =
41.30g.
50ml Distilled water
= 50g.
Read off against the Alcometric Table = 92% alcohol.
In this example the alcohol is not the requisite 95 %, and would need to be distilled again. Otherwise, unnecessary complications arise in preparing the official dilutions.
Alcometric Table 9.31A
|
SG 15�C |
% Vol. |
SG 15�C |
% Vol. |
SG 15�C |
% Vol. |
SG 15�C |
% Vol. |
|
0.815 |
95 |
0.877 |
75 |
0.924 |
55 |
0.959 |
35 |
|
0.819 |
94 |
0.879 |
74 |
0.926 |
54 |
0.960 |
34 |
|
0.832 |
93 |
0.882 |
73 |
0.928 |
53 |
0.961 |
33 |
|
0.826 |
92 |
0.884 |
72 |
0.930 |
52 |
0.962 |
32 |
|
0.830 |
91 |
0.887 |
71 |
0.932 |
51 |
0.964 |
31 |
|
0.833 |
90 |
0.889 |
70 |
0.934 |
50 |
0.965 |
30 |
|
0.836 |
89 |
0.892 |
69 |
0.936 |
49 |
0.966 |
29 |
|
0.840 |
88 |
0.894 |
68 |
0.938 |
48 |
0.967 |
28 |
|
0.843 |
87 |
0.897 |
67 |
0.939 |
47 |
0.968 |
27 |
|
0.846 |
86 |
0.899 |
66 |
0.941 |
46 |
0.969 |
26 |
|
0.849 |
85 |
0.901 |
65 |
0.943 |
45 |
0.970 |
25 |
|
0.852 |
84 |
0.904 |
64 |
0.945 |
44 |
0.971 |
24 |
|
0.855 |
83 |
0.906 |
63 |
0.946 |
43 |
0.972 |
23 |
|
0.858 |
82 |
0.908 |
62 |
0.948 |
42 |
0.973 |
22 |
|
0.860 |
81 |
0.911 |
61 |
0.950 |
41 |
0.974 |
21 |
|
0.863 |
80 |
0.913 |
60 |
0.951 |
40 |
0.975 |
20 |
|
0.866 |
79 |
0.915 |
59 |
0.953 |
39 |
0.976 |
19 |
|
0.869 |
78 |
0.917 |
58 |
0.954 |
38 |
0.977 |
18 |
|
0.871 |
77 |
0.919 |
57 |
0.956 |
37 |
0.978 |
17 |
|
0.874 |
76 |
0.922 |
56 |
0.957 |
36 |
0.979 |
16 |
The Official Dilute Alcohols 9.32
The dilute alcohols are similar in percentage strength in all
national pharmacopeias.
The strengths are based on scientific research evidence, relating to the
different solubility and decomposition characteristics of drug plant principles.
Accordingly, the drug plant monographs will state the solvent/menstruum strength for the various plant preparations, e.g. Tinctura Cinchona 70%, Tinctura Limonis 60%, Tinctura Quassiae 45%, Tinctura Myrrhae 90%.The monograph will usually state the upper and lower limits for the strength of the menstruum, which again, is given as v/v, e.g., Tinctura Digitalis 65 -70%. The lower level should not be below that given, lest the increasing volume of water commences to decompose the glycosides.
The official formula strength should be adhered to, in that way any small errors in reading a meniscus, or those caused by barometric or temperature factors, are minimized and no problems will arise.
Herbology deals with several hundred medicinal plants, for which no official monograph exist, and judgment must be exercised when selecting a suitable solvent for extraction. The student should become familiar with the taxonomic classification of those plants for which a monograph exists, i.e., the plants Genus and Specie.
The official monographs are representative of a genus or specie. Match the genus with that of the plant for which processing instructions are required. Plants of the same genus contain similar or related compounds, therefore, it is with a degree of confidence that the official monograph instructions may be followed. If, for any reason, a determination cannot be reached, then 80% v/v alcohol should be the solvent of choice.
Preparing the Dilute Alcohols 9.33
Distilled water is the medium of dilution.
Some pharmacopeias from the 1950�s onward use the term �Purified Water�.
That term takes into account the advances in water technology, sophisticated
filtering, and ion exchange, resins and so on. Many claims for the technology
are made, and many claims are unsubstantiated, it is convenient but expensive in
filter renewal, and as stated, question marks remain as to the water quality.
When diluting alcohol with water, remember that a contraction in volume occurs and heat is evolved. The adjustment to volume should not be made until the liquid is at 20�C. The figures given in the following Table show the volume of water that must be added to 100 volumes of alcohol, at the % shown.
Official Dilutions. Table 9.33A
|
|
95% |
90% |
80% |
70% |
60% |
50% |
45% |
25% |
20% |
|
95% |
- |
6.40 |
21.0 |
40.0 |
63.0 |
96.0 |
118.0 |
287.0 |
382.0 |
|
90% |
- |
- |
- |
31.0 |
53.5 |
85.0 |
105.0 |
266.0 |
355.0 |
|
80% |
- |
- |
- |
15.5 |
35.5 |
63.0 |
82.0 |
225.0 |
305.0 |
|
70% |
- |
- |
- |
- |
18.0 |
42.0 |
58.0 |
183.0 |
250.0 |
|
60% |
- |
- |
- |
- |
- |
21.0 |
35.0 |
142.0 |
200.0 |
|
50% |
- |
- |
- |
- |
- |
- |
11.5 |
100.0 |
150.0 |
|
45% |
- |
- |
- |
- |
- |
- |
- |
80.0 |
125.0 |
|
25% |
- |
- |
- |
- |
- |
- |
- |
- |
25.0 |
Distilled Water. General Points 9.34
The insistence of distilled water,
as opposed to water prepared by
treatment with ion exchange
resins, is not irrational, but
based on scientific research that has been carried out around the world. Alchemy
devoted a great deal of time to the study of water. With the rise of modern
chemistry, such alchemical notions were ridiculed, and water became the
simple chemical substance H2O.
However, modern atomic and atmospheric research has vindicated the alchemist. The implications for the life sciences of those findings, are only in recent years beginning to be understood. The indications are, that our bio-chemical concepts are seriously flawed.
Water is not a single substance with the simple formula of H2O, but rather a complex of substances which are not completely understood. Water contains another substance called Deuterium, which combines with oxygen to form the compound D2O, with an atomic weight of 2.016. It contains 1 proton, 1 neutron and 1 electron. Hydrogen has an atomic weight 1.008, and contains 1 proton and 1 electron.
Even the purest water contains six different isotopes, which can combine in 18 different ways, and it will contain no fewer than 33 different substances. Apart from removing unwanted solutes, the resins may be chemically altering the water in ways that are not readily detectable, which may set up a domino effect throughout a system, in which the water is part.
Preparation of Distilled Water 9.35
Freshly prepared distilled water
has an SG of 1.000, and
is neutral to litmus. When
water forms part of a preparation, it must be understood that the water means distilled water.
Water, irrespective of its source will contain impurities. These may be
soluble, insoluble, or as is more likely to be the case, both. The
impurities may cause chemical
reactions and unwanted physiological effects.
Distilled water is prepared from �normal� tap water, which may contain all or
some of the following impurities;
When water is heated to boiling, some of the substances contained are volatilized, and are collectively called pyrogens, which are usually gaseous, and will therefore pass through to the condenser with the first part of the distillate. Any dissolved solids will remain in the body of the still, however, due to a prolonged boiling, they may further decompose, releasing further pyrogens. Ammonia is a typical impurity, which is found in the first runnings of distillate.
Distilled water, produced on an industrial scale, and destined for non pharmaceutical use, such as vehicle batteries, is usually pre-heated to just below boiling point and held at that temperature for a number of minutes before being transferred to the still, where it undergoes a single distillation.
Distilled water for herbal preparations must be double distilled. The product from the first distillation is collected in fractions, i.e. on the assumption that we start with one litre of water,
the procedure is as follows;
1. The first 10% of the distillate is
rejected (100 ml).
2. The next 80% is collected, this is the
product. (800 ml).
3.
The final 10% (100ml) which contains non volatile contaminants, is left in the
still, and is rejected.
The yield is 800 ml. The water is then distilled for a second time;
A. The first 5% of the distillate is rejected
(40 ml).
B. The next 90% is collected, this is the
product. (720 ml).
C. The final 5% remains in the
still and is rejected. (40 ml).
The water should be stored in sealed glass containers until required for use.
-::-::-::-
Library
Pharmageddon
Herbal Block Index
![]()