
 Earth
Air Fire and Water
Earth
Air Fire and Water
The Pharmageddon Herbal
Chapter 5 Part 2.
Solar Heated Air Panel 5.22
Remember that Solar energy is the
substrata of all life. Solar Technology in one form or another has been used
since the dawning of our consciousness as specie. Today�s technology is
far beyond the price range of many people, couple that to the fact that the
repair of such sophisticated equipment is beyond the tooling capacity of
most of us. Therefore when I speak of it here, I do so as a passive system
which is owner built and is easily maintained from ones own resources.
When radiant energy falls upon the surface of an object, some is absorbed, some is reflected and some is scattered. Radiant heat obeys all the laws that govern light, and when it strikes an object some heat will be absorbed.
The efficiency of the heat transfer that takes place is governed by the density of a substance, the greater the density, the better the heat transfer efficiency. Remember that heat flows from hot to cold.
Solar air or water heating panels are built with these points in mind. Therefore, the thermal conductivity of an absorber plate is most important. Thermal conductivity will be briefly covered in Section 5.25. Solar air panels are simple to construct; the following schematic illustrates the principle.
Figure 5.22a
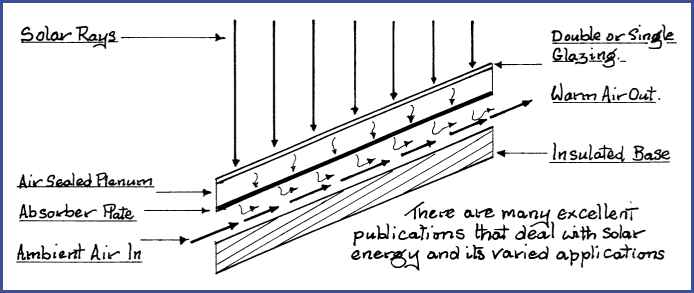
As the air moves through the collector, it strips the solar heat from the absorber plate and carries the heat with it. Highly polished surfaces are poor heat absorbers but very efficient reflectors, whereas a matt black surface is a good absorber but a poor reflector.
Wood Burning Furnace 5.23
A low-cost furnace can be constructed from
a 200 litre or smaller, oil drum. If a smaller size is chosen, remember that
it will be made from lighter gauge steel and will not last as long. A 200
litre drum, depending on how much use, and whether or not it was protected
from the elements, should last five or six seasons.
Suitable openings are made for ash pit, fire box and flue pipe exit. Fittings such as frames, doors and flue pipe collar can be made from suitable gauge steel and affixed with bolts for easy removal. The sheet steel should be thick enough to prevent a heat buckle. Gaps at contact points can be sealed with fire cement. The drum should be half lined with fire bricks laid on sand.
Fire Box Capacity is the volume of the drum used. The volume of a cylinder is measured by multiplying the area of its end by its length, i.e.
Radius x Radius x Pi x Length = Cubic Capacity.
The cubic capacity of a 200-liter drum is 200dm3. The Theoretical Heat Output is calculated as follows;
A. Average weight of wood = 550g/dm3 (Table 4.31A).
B. Average energy value = 10,000 kJ/dm3 (Table 5.12A).
C. Capacity of 200 litre drum = 200 dm3.
A x B x C = 1,100,000 kJ or 1.1 MJ.
Around 50% of the drum�s capacity cannot be used, due to the space occupied by the fire bricks, and the room required for fuel manipulation. Therefore, the theoretical output is 550,000 kJ. On the assumption of a 4-hour burn time, then the output is 137,500 kJ/hr. That is sufficient energy to evaporate 200 liters of water at 40�C.
Calculation of the Fuel Needed 5.24
For the sake of an example, assume 100,000
kJ/hr was required to dry a batch of the herb, then simply divide the energy
output required, by the energy output of the fuel chosen. In this example,
wood at 20,000 kJ/kg.
100,000 kJ/hr � 20,000 kJ/kg = 5 kg of wood per hour.
Thermal Conductivity 5.25
Heat always flows from hot to cold. The
rate at which the heat will flow depends upon the temperature difference
between two surfaces, eg., the absorber plate shown in Figure 5.22A, or the
temperature difference between the inside and outside surfaces of a wall.
The rate of flow will also depend on the type and thickness of the substance
through which it flows.
Now in consideration of Figure 5.22A, it will be understood that some very complex phenomena are being generated which overlap, fade and regenerate. An attempted detailed analysis would be futile and tedious. For our purpose, it is only necessary that we be aware that the process is constant.
The heat losses from a field scale dehydrator could be considerable. Therefore, a general rule must be employed, so that gross errors of calculation may be avoided. The values shown in Table 5.25A, are in watts per meter and may be considered as constants for dehydration purposes.
Thermal Conductivity Average Values. Table 5.25A
|
Material |
Range �C. |
Conductivity W/m2. |
|
Aluminium |
-5 to 220 |
220 |
|
Building Bricks |
-5 to 80 |
1.35 |
|
Concrete |
-5 to 80 |
1.75 |
|
Copper |
-5 to 220 |
385 |
|
Fire Bricks |
320 to 920 |
0.75 |
|
Glass Wool |
-5 to 80 |
0.035 |
|
Steel (carbon) |
-5 to 600 |
50 |
|
Steel (stainless) |
-5 to 220 |
20 |
|
Timber (pine) |
-5 to 80 |
0.15 |
|
Fire Board |
-5 to 650 |
0.15 |
Example; What is the heat flow through a brick dehydrator wall that is 6m x 2m x 150 mm?
Inside wall 40�C. Outside wall 15�C. The temperature difference = 25�C. Conductivity value = 1.35.
Area x Conductivity x Temperature difference � wall thickness.
6 x 2 x 1.35 x 25 � 0.150 = 2.7 kW
Fuel and Dehydrator Efficiency 5.26
Many factors contribute to the loss of
heat energy during the dehydration process. A major factor is an
inexperienced operator, who, by a simple error could ruin a dehydrator load
of herb. The experience is gained during dehydrator trials with foraged
biomass materials. A competent operator will have a thorough grasp of
dehydration theory that will allow that person to relate to, and modify
dehydration phenomena as it unfolds.
Planetary, lunar and solar cycles are intimately bound to the business of herbs; but let it be clearly understood that the only cycle that applies to herbal dehydration, is the bicycle i.e. efficiency and speed. From harvest point on, the herb is dying, and a quick clean death is required. It will be understood that considerable disparity between theoretical and actual performance of dehydration apparatus is possible. It is essential that a grower has an accurate set of figures from which to work, if success is to be achieved.
The efficiency of a dehydrator can only be determined by actual drying trials; from which the amounts of fuel burnt and water evaporated, can be expressed as a percentage of dehydrator efficiency i.e.
Kg fresh herb - kgs dried herb � kgs fuel burnt x heat value of fuel = Dehydrator efficiency
To drive a dehydrator, at maximum efficiency is a skilled job, therefore, drying trials and the figures derived from the trials, must accurately reflect efficiency and competence of the operator. As a rule an operator should undertake a minimum of a weeks training, consisting of not less than 12 dehydration trials, to ensure competence. The efficiency trial should consist of not less than 3 separate trials for each type of material i.e. root, leaf, whole herb and flowers. An average for each type of material is used to determine efficiency.
An example of a dehydrator log book is given in section 5.40. For the purpose of example let us assume that we have extracted the following figures from the log book;
|
A |
Weight of fresh herb |
100 kg |
|
B |
Weight of dried herb |
25 kg |
|
C |
Drying ratio |
4:1 |
|
D |
Weight of fuel burnt |
8 kg |
|
E |
Heating value of fuel |
20,000 kJ |
|
F |
Latent heat of vaporisation at 40�C |
2410 kJ/kg |
Therefore, A � B = 75kg evaporated water.
D x E = 160,000 kJ of energy used.
75 � 160 = 0.468 = 46.8% efficiency.
A dehydrator that could return 55 to 60% would be exceptional. A suitably sized solar panel would increase efficiency.
Obviously, such figures take no account of the energy needed to bring the dehydrator up to working temperature, but they do reflect dehydrator losses. Losses can be minimized and will be covered later in the text.
Estimating Dehydrator Capacity 5.27
The herb growers dehydration capacity must
match the amount of herb grown if economic loss is to be avoided. The first
step in plantation establishment, is to decide what species of herb is to be
grown, and how much of each. When those questions have been decided the
grower should then produce a dehydration schedule. You are referred to
sections 2.22, 2.23 and table 2.23A.
For the purpose of an example, it will be assumed that a 1 hectare cultivation has been decided on and allocated to crops, as per Table 2.23A.
A grower will understand quite clearly that each day that a mature crop stands in the field is a calculated risk, therefore, speed is of the essence. The Table will show that midsummer is the critical period; and that there are two periods of specie overlap. Chamomile being the overlap specie. If harvesting is undertaken with care, then chamomile will provide two or three cuts of mature flower per season. It will also be seen that lemon balm provides a greater fresh yield than catnip. Therefore, lemon balm and chamomile are the two species upon which dehydration capacity will be determined.
Column �C� shows that a total of 1386 m� of drying space is required to process the crop. If the figure is divided by the harvesting period i.e. seven days, then 198 m� of drying space must be provided on a daily basis. That figure is then divided by the number of dehydration runs per day. If the dehydrator has a capacity of continuous running, then three loads per day would not be excessive, i.e. 66 m� of drying space must be provided to handle the crop.
On the basis of moveable 1 m� drying trays, that are stacked with a gap of 18 cm between each tray (see Figure 5.20B and 5.20C), then we have the following; 66 x 1 m� x 0.18= 11.88 m� or 12 cubic meters of space.
Therefore, the dehydrator will need to have a capacity of 12 m�, to meet requirements. It should be clearly understood that the figure arrived at does not represent a constant for all specie, because tray loading capacity depends on the type of material it holds, i.e. root, herb or flower.
When estimating dehydration capacity, a prudent grower would allow for 20% over capacity. The increased running costs would be insignificant if placed along side cost of under capacity.
Selection of Dehydrator Apparatus 5.28
The plantation and the processing facility
must be laid out on an ergonomic pattern. Do not under estimate the labor
involved in raising and transporting the crop to the processing facility.
A fully loaded 12 m� dehydrator contains 0.25 tonne of root, or 0.20 tonne of herb. Do not under estimate the time and labor involved. If the wrong type of dehydrator is selected, it will impact on every aspect of the operation. The dehydrator is the bottle neck through which everything must pass at a time when the grower is most vulnerable to loss. Think the process through!
As an owner built dryer, the tunnel and the trolley method cannot be matched; technically or ergonomically. It is simple to drive and its capacity quickly and cheaply expanded to match an increase in cultivation. The design allows the grower to use parallel or counter flow methods, whilst the rate controlled air return duct enables the operator to produce a wide range of dehydration climates.
Calculating Energy Requirements 5.29
Energy requirements for a given
dehydration process will depend upon the following factors;
A - The temperature of the atmospheric air.
B - The size and thermal conductivity of the dehydrator.
C - The amount of water to be evaporated.
D - The temperature at which the evaporation occurs.
Factor A is a variable. A normal temperature reading of the atmospheric air is required.
Factor B is a variable dependent on temperature. See Section 5.25.
Factors C and D are limited variables. See Table 3.11A.
Lets deal with each factor in turn;
Factor A. The temperature of the
atmospheric air is only important as a reference point when set against the
drying temperature of the herbal material. If the atmospheric air
temperature is subtracted from the drying temperature, we are left with a
figure that gives the number of �C that must be provided for if the
dehydration is to proceed in a satisfactory manner e.g.
Ambient air temperature 8�C
Dehydration temperature 60�C
Temperature difference 52�C
Therefore, sufficient heat must be added to raise the air temperature by 52�C.
For the purpose of example, all the subsequent calculations will be based on standard temperature and values (StV) Quite obviously, the lower the ambient air temperature, the more fuel must be burnt to maintain operational dehydration temperature.
Factor B. There are numerous points that must be taken into consideration. A dehydrator constructed in part, or entirely from combustible materials, represents a fire risk. If the dehydrator burns out during harvesting, then the crop standing in the field is also lost. A fire risk may well be impossible to insure, or premiums so high that the process becomes very expensive. Therefore, expensive fire proof insulating materials must be resorted to, or the thermal conductivity of the material so high, that thermal loss is unacceptable, e.g., sheet metal of roofing iron constructions, which also suffer from condensation and corrosion problems.
Accordingly, it makes sense to construct the main structure from materials that meet those problems, ie, bricks, concrete blocks, soil/cement bricks or adobe. From a do-it-yourself aspect, soil/cement bricks will meet all the criteria. They may be easily made on site by using a Cinva Ram and do not require firing; the total cost is a fraction of commercially made alternatives. A perusal of Table 5.25A will help clarify the points made. Therefore, if those suggestions are followed, then for practical purposes, heat loss calculations may be dispensed with. However, it must be remembered that energy must be expended to bring the apparatus up to operational temperature.
Factor C. The amount of water that must be evaporated to complete the drying process will depend upon the type and amount of herbal material being operated on. For example, 100 kg of fresh herb contains around 75 liters of water and flowers contain around 90 liters. It will be remembered that water has a specific heat capacity of 4.2 kJ/kg, therefore, it will require 4.2 kJ of heat for each liter of water to raise its temperature by 1�C.
For practical purposes it may be assumed that the herbal material is at the temperature of the ambient air, which at StV, is 15�C. If the drying temperature is 40�C, the temperature difference is 25�C. Accordingly, 4.2 kJ must be multiplied by the temperature difference to give the amount of heat needed to evaporate 1 kg, or liter of water at 40�C. This amount of heat must be supplied constantly until the evaporation is complete.
Factor D. The latent heat of evaporation is a function of the wet bulb temperature of the air in contact with the herbal material. That relationship may be expressed as follows;
Enthalpy of Evaporation Figure 5.29A
For the sake of simplicity, the graph is not strictly accurate to decimal places but it may be used with confidence for dehydration purposes.
The Heat Calculation 5.30
The behavior of herbal material under a
dehydration regime is very close to that of a free water surface, and in all
probability is linked to the thickness of the material. To maintain that
favourability with a root crop, it should not be sliced at more than 50 mm
thick.
The total heat required is calculated in three steps.
1. The heat required to bring the dehydrator up to working temperature, this will depend on the temperature of the atmospheric air and the thermal conductivity of the dehydrator. In practice, this figure may be derived from the dehydrator trials by noting the amount of fuel required to bring the dehydrator up to operational levels.
2. The temperature difference between the ambient and operational temperature is noted, e.g. StV is 15�C. Operational temperature for herb is 40�C.
Temperature difference is 25�. The specific heat capacity (SHC) of water is 4.2 kJ/kg. See table 4.31A
25 x 4.2 kJ/kg = 105 kJ/kg required to raise the temperature from 15�C to 40�C for 1 kg of water.
On the assumption that 100kg of fresh herb contains 75 litres of water the calculation is as follows;
75 x 105 = 7875 kJ of heat required to raise the temperature of the herb to 40�C.
3. The heat required for the evaporation of the water at 40�C is 2420 kJ/kg (See Figure 5.29A) Therefore the rate at which the heat must be supplied to effect the dehydration in 1 hour, is calculated as follows;
Water for evaporation 75kg. 1 hour drying time in seconds 3600
75 � 3600 x 2420 = 50.41 kW
Therefore to complete the drying process in 1 hour the heat energy required is 50.41 kW/hour, divided by the appropriate drying time. On the assumption of a 4 hour drying time the calculation is as follows;
50.41 kW/hr � 4 hours = 12.6 kW/hr.
N.B. Such figures take no account of the effciency of the combustion process or heat loss from the dehydrator.
Calculation of Air Volume 5.31
The volume of air passed through the
dehydration chamber must be sufficient to remove the water vapor liberated
from the herb. Air volume will vary according to temperature.
Variation in Air Volume Figure 5.31A
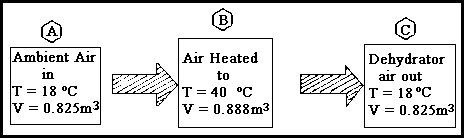
The scale is 1:40. The volumes are extracted from the psychrometric chart.
Air volume figures will be greatly simplified if you calculate in weight, and then convert to volume as the final calculation. By heating the air, the volume, and therefore, its moisture holding capacity, has been increased. Accordingly, the temperature drop, when the air leaves the dehydrator, it is a measure of the work done, i.e. the amount of water vapor removed. Figure 5.31A represents the ideal, which in practice, would be very difficult to obtain, because of the large number of variables introduced within the dehydrator. However it is sufficient to understand the raw physics involved.
If you look at Figure 5.29A, it shows that the enthalpy, or latent heat of evaporation at 40�C, is 2420 kJ/kg; that figure also represents the volume of air in kilograms that is needed to evaporate 1 kg of water, with a temperature drop of 1�C on leaving the dehydrator.
Accordingly, if the air leaves the dehydrator at 25�C, i.e. a 15�C drop in temperature, then the following calculation may be arrived at;
Kg of air dropping by 1�C � Temperature drop of 15�C = kg/air
2420 � 15 = 161.33 kg of air.
A glance at the psychrometric chart will show that air, 1 kg at 40�C, has a volume of 0.888 m�.
Volume x weight = m� of air
0.888 x 161.33 = 143.26 m� of air
From that figure, we may then ascertain the air velocity, or flow rate, needed to evaporate 1 kg of water in one hour
143.26 � 60 minutes = 2.38 m� of air per minute
2.38 � 60 seconds = 0.039 m� per second
Remember that 1 m� = 1000 litres, the flow rate required is 39 litres per second.
Commercial fans are rated at the number of litres of air that they can move in one second against a given pressure.
Estimating Fan Power 5.32
You will not know in advance how efficient a dehydrator will be when built. Nonetheless, it is essential to know what the fan power should be under a given set of conditions. The conditions set must represent the extremes of the conditions to which your dehydrator must function. Accordingly, you must assemble the following information;
1. The drying space required to handle the crop.
2. The cubic capacity of the dehydrator.
3. Lowest temperature encountered during operations.
4. Drying temperature.
5. Allotted drying time.
6. Heat loading required.
7. Air volume needed to carry the heat and remove vapor.
For the sake of example, the following information is assembled;
A. 66 m� i.e. 66 x 1 m� trays stacked at 18 cm intervals.
B. 12 m� based on 66 x 1 m� trays holding 300 kg of root.
C. Mid autumn average temperature 5�C.
D. 60�C, based on a root crop with 200 litres of water to be removed.
E. Seven hours, based on the root crop.
F. The heat loading is calculated from the above data.
G. The air volume is calculated from the above data.
The above information is a variable, which is determined by your own situation, i.e. size of cultivation, type of crop, geographic location. The temperature difference between ambient and operational in this example is 55�C.
Temp diff x SHC water x water mass = kJ
55 x 4.2 x 200 = 46,200 kJ
That is the amount of energy needed to raise the temperature of the root, to operational temperature of 60�C.
The enthalpy, or latent heat of evaporation at 60�C, is 2380 kJ/kg (see Figure 5.29A).
Enthalpy/latent heat x water mass = kJ
2380 x 200 = 476 000 kJ
To that figure we must also add the energy needed to raise the root to operational temperature of 60�C.
476,000 kJ + 46,200 kJ = 522200 kJ
The total energy required is divided by the assumed temperature drop to arrive at the number of kg of air required to carry the heat needed to dry the root in 1 hour.
522200 kJ � 15�C temperature drop = 34813 kg/hour
The kg of air required are then divided by the target drying time. For root it is 7 hours.
34813 � 7 = 4973 kg/hour
Therefore 4973 kg air/hour is divided by 3600 seconds which is the number of seconds in one hour.
4973 � 3600 = 1.38 kg/second.
Now convert to air volume. The psychrometric chart will show that air at 60�C has volume of 0.940 m�.
1.38 x 0.940 = 1.2972 m� of air per second.
Round the figure and we have a fan rating of 1,300 litres per second. It would be prudent to allow for 1500 litres per second. If you plan to expand a cultivation, bear that in mind, when purchasing fans.
Library
Pharmageddon
Herbal Block Index
![]()