
 Earth Air Fire and Water
Earth Air Fire and Water
The Pharmageddon Herbal
Chapter 4 Part 3
Macroscopic and Microscopic Quantities.
4.24
Physics may be called the wholistic
science, the science of everything, and from which, every other branch of
science must draw sustenance.
Physics seeks to link each component of the known universe; and further to link the macroscopic quantities and properties of each component to its microscopic counterpart. It was by employing microscopic or reductionist methods, that physics was able to demonstrate the quintessential unity of the macrocosm, i.e. the Hermetic viewpoint.
The intellectual journey from concept to demonstration was a tour de force of human reasoning power, from which stream a multitude of benefits.
The concept of time allows us to make sense of the profusion of natural phenomena that surrounds us. It is fundamental to all of our activities, and the greatest degree of accuracy is obtained, by measuring microscopic quantities. Example, the S.I. Unit for time is the �second� (s).
An instrument to measure time is the clock. Around the year 1900, the best degree of accuracy that we possessed was a clock that only lost 1 second every three months, ie, an accuracy of 0.01 of a second. 103 years later we are able to measure time with the so called atomic clock, whereby time is measured against the vibratory rate of the cesium atom (see Periodic Table. Atomic number 55), which only gains 1 second in 27,000 years, an accuracy of 0.0000001 of a second.
For the purpose of dehydration we need to work with the macroscopic concepts of time , temperature, pressure, volume and energy; therefore, it is an aid to the understanding of the base units used, if it is borne in mind that each base unit is linked to the microscopic quantity and property.
Work 4.25
Pushing a stalled vehicle in order to
start it is a common sight. The person pushing the vehicle is applying
force, to make the vehicle move, ie, they are doing the work.
The amount of work done is measured by multiplying force, by the distance through which it travels.
The unit of force is the Newton (N). A unit of
work is formed from the units of force and distance, the unit is the Newton
/metre, which is called the Joule. (J).
Power 4.26
Power is defined as the time rate at which
work is done. The S.I. Unit for power is the Watt (W). One watt equals 1
joule per second (1 J /s), therefore, 1W is the force of 1 Newton moving
through a distance of one metre in 1 second. (N/m/s).
Energy 4.27
Anything which is able to do work contains
potential or stored energy, eg, a piece of coal is potential heat which is
released when it is burnt; in other words, the energy is released by
initiating a non reversible chemical change (see section 5-8).
Energy can be classified into 3 types, which are;
(1) Potential energy, eg, a piece of wood, an electrical battery or water stored behind a hydro-electric dam.
(2) Heat energy (refer. Section 4.10) is connected to the vibratory rate of atoms and molecules, therefore, heat energy is a potential of all matter, e.g. burning of fossil fuels.
(3) Kinetic energy is the potential energy of a body in motion, eg, the arm drives the cue, which strikes the cue ball, which strikes the pool or snooker ball. At each point of impact, the velocity or potential energy, is converted to kinetic energy, which transfers, in part to the object struck, i.e. a kinetic exchange occurs.
A more relevant example would be that of warm dry air passing through a dehydrator; on entering it contains more heat and less moisture. Heat is taken up by the herb, which releases moisture to the air. Therefore, the air on leaving contains less heat and more moisture, than on entry. A kinetic change has occurred.
Thermodynamics 4.28
Thermodynamics is a branch of science that
deals with the relationship between heat and other forms of energy.
The principle of the conservation of energy states that energy can neither be created or destroyed, but is convertible from 1 form to another e.g. heat and mechanical energy are mutually convertible, therefore 1 joule of work equals 1 joule of heat. However, during the process of conversion there are losses of energy, due to inefficiencies in the system of conversion.
The energy is not really lost but has been converted to a form that is not useable, eg, a vehicle losses much heat energy through its hot exhaust gas. The efficiency of any conversion system may be known by the following expression;
Output � Input = efficiency
Example; Output 1 � Input 2 = 0.5 efficiency.
A conversion system that can better a 50% efficiency rate is exceptional rather than the norm. Therefore, according to the thermodynamic theory of entropy, the unusable energy is dissipated; creating an increasing molecular disorder, which will eventually lead to the heat death of the universe; in that heat moves only from hot to cold, and eventually there will be temperature equilibrium throughout the universe.
The first and second laws of the thermodynamics are subject to much controversy *. With tongue in cheek we may say, " There is some pressure on the matter". For our purpose the situation can be summed up, by Michael Flanders and Donald Swann�s rendition of a little ditty;
You cant pass heat from a cooler to a hotter. Try it if you like, you�d far better notter, cause the cold in the cooler will get hotter as a ruler, cos the hotter bodies heat will pass to the cooler. Oh you cant pass heat from a cooler to a hotter. Try it if you like, you�d only look a fooler. Cold in the cooler will get hotter as a ruler, that is a physical law. Heat is work and work�s a curse and all the heat in the universe is gonna cool down because it cant increase, there�ll be no more work and perfect peace;
Really ? Yeah, that�s entropy man!
�all because of the second law of thermodynamics�
At the Drop of Another hat� E.M.I. Records 1964
*
The second law of thermodynamics contradicts the findings of cosmology and evolution. This of course presents a problem for Science which has not been properly addressed, and never more so in the field of medicine and the periodic table."As far as the laws of
mathematics refer to reality, they are not certain, and as far as they are
certain,
they do not refer to reality".
Albert Einstein
Heat and Temperature 4.29
There is a tendency to talk of heat and
temperature as though they were one and the same thing; there is however, a
critical difference. If we refer back to the theory of matter, then the
following statements may be put in context;
A. Temperature is a measure of the vibratory speed of an atom.
B. Heat is the vibratory speed of an atom multiplied by the mass of atoms.
Therefore 2 litres of water at 25�C contains more heat than 1 litre of water at 25�C.
Heat Energy 4.30
Units of heat are measured in Joules; and
when working on macroscopic quantities, it is usual to work in kilo-Joules
per unit of mass ie kJ/kg.
Heat energy can be a difficult concept to understand; for unlike matter it cannot be seen or held, however, its effects can be felt.
For example, assume that we have 1 kg each, of Ethyl alcohol, olive oil and distilled water, at a temperature of 25�C.
To each one we add 5 k/J of heat energy, then from the initial starting temperature, the ethyl alcohol would register 27.04�C, a rise of just over 2�C. The olive oil would register 27.5�C and the water 26.19�C.
If we applied the same conditions to a block of copper, the temperature would register 37.56�C, a rise of 12.56�C. Quite clearly different substances vary greatly in their temperature reaction to heat energy. The reason is because of the difference in density or atomic mass of the different substances (see Periodic Table), therefore, it is not possible to use temperature as a measure of heat without first specifying the substance which is being heated.
Specific Heat Capacity 4.31
1 litre of water at 4�C weighs 1 kg and
has a volume of 1 cubic decimetre (1000 cubic centimetre), 1dm3
of copper weighs 8.79kg.
Now although the volume is the same, there is a big difference in mass, therefore, the copper and water will require different amounts of heat energy to lift the temperature by 1�C.
Each substance has its own specific heat capacity which is measured in kJ/kg/�C which is the amount of heat energy needed per kilogram of substance to raise the temperature by 1�C.
The term specific heat capacity is a misnomer, because it implies that a substance will only hold a certain level of heat; whereas in fact, the S.H.C. differs with pressure and temperature, and by pouring heat energy into a substance, we can produce the following effects;
● An increase in temperature (per kJ/kg �C)
● Expansion of volume (size)
● A change in physical state (solid, liquid, vapour)
● A chemical change (burnt toast)
● A change in the electrical properties of the substance.
The specific heats of different substances were arrived at experimentally. In the terms of the old heat units, the specific heat of water was unity or 1.
The specific heat capacity of the other substances were then related to water, the quantity given was a ratio, and was not a heat unit.
Under the S.I. the quantities are heat units related to the mass, and not a ratio related to water.
Energy in whatever form costs money, therefore, unit operations whether laboratory or field scale, must be taken into the economic costings if any degree of accuracy is to be obtained. You must know the amount of heat required to bring a dehydrator, evaporator or distilling unit up to working temperature, and then the amount of heat required to carry out the process.
Specific heat capacities for solids will vary according to temperature. Specific heat capacities for gas and liquids will vary according to temperature and pressure. The values which are given in Table 4.31A, are average values which, for all practical purposes, may be taken as constants.
Density and Relative Density 4.32
The density of a substance is defined as
its mass per unit of volume, e.g. kg/m3, kg/dm3, or
g/cm3.
The density of water is greatest at 4�C. At that temperature, 1000 litres or kilograms has a volume of 1 cubic metre or 1000kg/m3. Therefore, it follows that 1 gram has a volume of 1 cubic centimetre at that temperature.
The specific heat values are expressed as kJ/kg, therefore, if you know the weight of a dehydrator or distillation unit then it is simple to calculate its S.H.C.
The relative density of a substance is a ratio of distilled water at 4�C under pressure of 1 atmosphere. The densities of selected substances are given with the S.H.C. values in Table 4.31A.
SHC and Densities. Table 4.31A
|
Comparative Substance |
Density kJ/kg |
Density Kg/m� |
Density g/cm� |
Relative Density |
|
Air (STP*) |
1.000 |
1.30 |
0.0013 |
0.0013 |
|
Aluminium |
0.880 |
2720.0 |
2.72 |
2.72 |
|
Brass |
0.377 |
8480.0 |
8.48 |
8.48 |
|
Concrete |
0.440 |
1902.0 |
1.90 |
1.90 |
|
Copper |
0.398 |
8790.0 |
8.79 |
8.79 |
|
Iron (cast) |
0.530 |
7200.0 |
7.20 |
7.20 |
|
Sand |
0.453 |
1294.0 |
1.29 |
1.29 |
|
Steel (carbon) |
0.481 |
7820.0 |
7.82 |
7.82 |
|
Steel (stainless) |
0.510 |
7900.0 |
7.90 |
7.90 |
|
Stone (average) |
0.465 |
2200.0 |
2.20 |
2.20 |
|
Water |
4.200 |
1000.0 |
1.00 |
1.00 |
|
Wood (average) |
1.210 |
556.0 |
0.556 |
0.556 |
STP = Standard temperature and pressure. STP = 0�C at 101 kPa. (Freezing point at sea level)
The subject of pressure will be dealt with later in the text.
Heat Tranfer 4.33
In section 4.28, Michael Flanders and
Donald Swann in their rendition of the 2nd Law of Thermodynamics,
inform us that, �you can pass heat from a cooler to a hotter�; in other
words, heat always flows from a hot substance to a cooler substance. Heat
will continue to slide down the temperature gradient between hot and cold
until equilibrium or equal temperature exists between the two substances.
This phenomenon is very useful because we can move heat energy from one place to another by arranging a series of temperature slides. Heat can be transferred in three ways;
1. By Conduction. E.g. we can go to bed in a warm house and wake up to a cold house. The heat has diffused through the walls and dissipated into cold night air, there has been a temperature slide between the house, and the atmosphere. We use that principle when warm air is passed across cold herb.
2. By Convection. This method of heat transfer is achieved by using a gas or a liquid as a vehicle for the heat energy, e.g. a domestic fan heater draws air across an electrically heated element. Heat is transferred to the cool air, which becomes warm, and is then blown into the room. We use that principle when air is heated for dehydration purposes. The use of liquids for heat transfer finds many uses, for instance, water or steam radiators, or domestic refrigerator.
3. By Radiation. Unlike conducted or convected heat, radiated heat may pass through a vacuum. It does so as a wave motion which is similar to that of radio or light waves. Radiant heat obeys the same physical laws that govern light. That fact is the core principle of Helio-technology. That will be expanded when solar energy is discussed.
Thermometers and Temperature 4.34
A thermometer (thermo- meter) is an
instrument that utilises the thermometric properties of expansion and
contraction, to measure a temperature change to a substance. The most common
type is one that uses the expansion and contraction of a liquid to measure
the heat intensity of a substance.
Water is not suitable because it freezes at 0�C. The liquid chosen must have a lower freezing point than water, eg, alcohol and mercury (Hg).
There are 2 fixed points against which temperature scales may be calibrated, ie, the freezing and boiling points of water at a pressure of 1 atmosphere. (The subject of pressure will be covered later in the text) If the pressure on water is over 1 atmosphere, the boiling point will be raised and the freezing point lowered,. below 1 atmosphere, the boiling point is lowered and freezing point raised.
Temperature Scales 4.35
In the past, there were a variety of
temperature scales in use in different parts of the world, most of which are
now abandoned. There are now three international scales recognised by
science and technology, they are;
1. The Celsius Scale, which was formerly called �Centigrade�. This is the scale that is used for most practical purposes, and the fixed points are determined at 1 standard atmosphere, i.e. 101.325 kPa. (Sea level).
The lower fixed point is the fusion or freezing point of water, i.e. 0�C. The higher fixed point is the boiling point (bp) of water, i.e. 100�C. The interval between the 2 points is divided into 100 divisions, each one representing 1�C.
2. The Thermodynamic or Kelvin Scale, This is the fundamental scale to which all temperatures are finally referred. The scale is not allied to any substance, and to avoid confusion the term �degree� is not used, instead the term Kelvin is used, e.g. 373K = 100�C. To give a scale a numerical basis, the scale is compared to the �Triple Point of Water� (tp). The triple point of water is the equilibrium point between ice, water and water vapour, i.e. 0�Celsius.
That point on the Kelvin scale is 273.15K or 273K for practical purposes. To convert from Celsius to Kelvin, add 273, e.g. 100�C + 273 = 373K. To convert from Kelvin to Celsius, subtract 273 e.g. 373K - 273 = 100�C. The Thermodynamic or Kelvin scale postulates an absolute zero, or 0K, and because the Kelvin and Celsius use the same divisions, 0K = 273�C.
3. The International Practical Temperature Scale, or the IPTS was legally adopted to solve practical problems that were involved with calibration of industrial and scientific instruments that are used in the areas of cryogenics (very low temperatures), or in pyrogenic work such as may be involved with thermo-nuclear reactors or heat shields for space craft.
The scale is in �C, and for practical purposes can be considered identical with the Kelvin scale. There are various fixed points which, for our purpose are irrelevant, but to convey the idea, one is based on the fusion or melting point of gold, i.e. 1064.43�C, and another on the boiling point of oxygen, ie, -182.962�C.
Comparison of Temperature Scales Table 4.35A
|
Water |
Kelvin |
Celsius |
Fahrenheit |
|
Boiling point |
373K |
100�C |
212�F |
|
Standard point |
293K |
20�C |
68�F |
|
Triple point |
273K |
0�C |
32�F |
|
Absolute zero |
0K |
- 273�C |
- 460�F |
To convert Fahrenheit to Celsius; Subtract 32�F, multiply the answer by 5, then divide by 9 = �C
Example, 212�F � 32 = 180 x 5 = 900 � 9 = 100�C.
Energy and Change of State 4.36
Figure 4.11A is a representation of 3 of
the states of matter, ie, Solid, Liquid and Vapour; each particle represents
a molecule. In the solid the molecules are tightly bonded by
electro-chemical force. In the liquid state the bonds have been stretched
and weakened, which allows for a certain degree of movement. The vaporous
state shows that the molecules have broken free from the electro-chemical
energy that bound them together.
In order to bring about a change of state in water molecules, sufficient force, or heat energy, must be applied to the body of water to weaken or break the electro-chemical bonds that bind the molecules together. The state of a substance depends partly on temperature and partly on pressure.
Sensible Heat 4.37
If we take a pan of cold water and place a
thermometer in it, the temperature of the water may be seen from the scale;
remember that, we are not measuring the amount of heat in the water, but the
heat intensity of the water.
If we then place the pan of water on a heat source and observe the thermometer, it will become obvious that although we are pouring heat energy into the water, the temperature does not change.
However, as we continue to observe, after a period of time the temperature will start a steady climb, so that we can see the effect of the heat energy. Heat that brings about an observable change in the temperature is called sensible heat.
The time lag between the heat poured in, and the initial temperature rise will vary from substance to substance, i.e. the differing specific heat capacities, and also the rate at which the heat is poured in, and the starting temperature.
Heat and Change of State 4.38
The point at which a solid will change to
a liquid or a liquid to a solid, e.g. the freezing or melting of a ice cube,
is known as the temperature of melting or the temperature of fusion.
The point at which a liquid turns to vapour is the temperature at which the substance will boil and vaporise.
When a substance is undergoing a change of state the temperature will remain steady until the change of state is complete. (Refer to Section 4.12). In the past the heat that was being added or taken away from a substance without a change in temperature was called latent heat.
The amount of heat required to bring about fusion or vaporisation in any substance is different, so there was a latent heat of fusion and a latent heat of vaporisation.
Today latent heat is called �Enthalpy�, therefore, there is enthalpy of fusion and enthalpy of evaporation of a substance.
For our purpose, we will take the terms of evaporation and vaporisation to be synonymous; but it must be borne in mind that water will evaporate at all temperatures above freezing. The relationship between sensible heat and enthalpy heat may be understood by studying the chart in Figure 4.38A.
Enthalpy. Temperature. Change of State Fig. 4.38A
The following values are for fresh water at 101 kPa (Sea level)
Specific enthalpy of fusion ( Ice ) ------------ 335 kJ/kg
Specific heat of water ----------------------- 4.2 kJ/kg
Specific enthalpy, vapourisation of steam ---- 2260 kJ/kg
The chart represents 1kg of ice through to vapourisation.
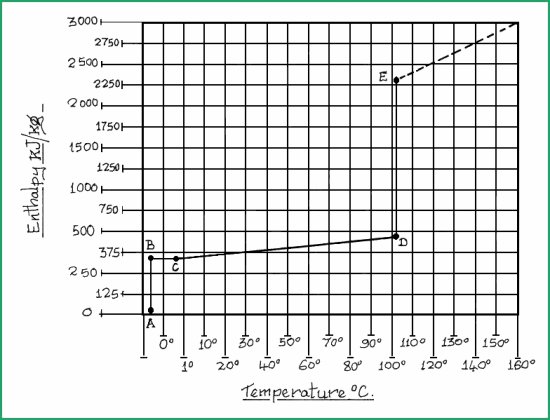
Explanation of the chart.
Point A to B = 335kJ of heat added with no rise in temperature.
Point B to C = Ice melting with no rise in temperature.
Point C to D = Water temperature rising by 1�C for every 4.2kJ/kg
Point D to E = 2260 kJ/kg of heat added without a rise in temperature to vapourise the water
C = Melting point. A. B. C = Latent or hidden heat
D = Boiling point. C. D. = Sensible heat (temperature rise)
E = Vapour point D. E. = Latent heat
Atmospheric Pressure 4.39
The concept of pressure is of importance
in many areas of herbology, as it relates to unit operations. For example,
pressure or lack of it, determines the fusion or vaporisation point of a
substance; also we make use of the pressure set up by a fan to move air in
the unit operation of dehydration.
Fish live in an ocean of water. Most people will understand water (hydrostatic) pressure, i.e. the deeper one descends, the greater the pressure on the body, with the ear drums being particularly sensitive.
Terrestrial creatures live at the bottom of an ocean of air and the pressure is considerable. Pressure may be defined as a force per unit area on an object, e.g. 1 atmosphere exerts a pressure of 1.03 kg/cm2. The average adult has a surface area of 19,500 cm2, i.e. the sum of the pressure on the body exceeds 19 tonnes, or the equivalent of a 10 metre depth of water.
Vapour Pressure 4.40
If we take a container of cold water and
bring it to the boil, it will be seen that small bubbles begin to form on
the inside of the container.
As we continue to pour heat energy into the water, the vapour bubbles become larger and more numerous. The water that surrounds a bubble starts to vaporise into the bubble, which expands, the expansion exerts a vapour pressure. Many text books generalise by stating that a liquid will boil when the vapour pressure is at equilibrium with the pressure upon its surface.
A discussion of the gas laws is inappropriate for this text, but if you think about it, a more accurate definition would be , that water will boil when the vapour pressure of the bubbles reaches equilibrium with the water, or hydrostatic pressure. That definition makes it easier to understand how water can evaporate from a free surface at all temperatures above freezing.
The Effect of Increased Pressure 4.41
If we take a sealed vessel of water and
pump air into it, there will be an increase of air pressure on the surface
of the water. That makes it more difficult for the water molecules to escape
as vapour from the water surface, and will also increase the hydrostatic
pressure.
Therefore, a greater amount of heat energy will be required to enable the vapour pressure to overcome the increased pressure upon it. Logically, the increase in heat energy will cause an increase in the temperature; therefore, the boiling point of a liquid will rise with an increase in pressure.
Refer again to Figure 4.38A and place a ruler along the �C�, �D� line so that the ruler intersects the 160� temperature line. The line �D�, �E� will then be at a higher point on the graph, i.e. the boiling and vapour points have been increased.
The Effect of Reduced Pressure 4.42
If we now take the same vessel of water
and attach a vacuum pump to it and reduce the air pressure to a point below
atmospheric, we will also reduce the hydrostatic pressure. The reduced
atmospheric pressure enables water molecules to leave the water surface more
readily, in other words the vapour pressure is higher than the overlying air
pressure.
The reduced hydrostatic pressure will enable the water to boil at a temperature below 100� C. The point of equilibrium where the number of water molecules escaping balances the number of molecules condensing, is known as the vapour pressure of the liquid, or to be precise, � the equilibrium vapour pressure�.
This phenomenon is used to advantage by the Herbologist. Herb metabolites are thermo labile, i.e. damaged by heat; but by operating on the body of the herb under reduced pressure the Herbologist is enabled to extract metabolites without damage.
Library
Pharmageddon
Herbal Block Index
![]()