

Earth Air Fire and Water
The Pharmageddon Herbal
Chapter 4
Introduction.
There is a great mystery in numbers. Five
digits on one hand, and two eyes in the head. The mysterious figure 7 again.
Hands and eyes in the service of the brain produce the number 3. The great
Triad of Nature from which, all of our human ambitions and artifacts issue
forth.
It was with number that those ancient sages divided the vault of the heavens and thus regulated the bounty of nature. Without numbers, time and space would be incomprehensible to our kind. From numbers came a rickety space station. From number, came the Pyramids and the great Cathedrals. From number came skyscrapers and mighty dams to attempt to harness the power of nature. Our very technology rests on numbers. Where would we be without numbers ?
Fundamental Concepts 4.1
For many of us the mere mention or sight
of �mathematical formulae� tends to produce a mental cringe and scuttle,
therefore, all such formula have been reduced to simple, basic arithmetic.
The larger numbers may be quickly and easily handled with a simple pocket
calculator. The scientific concepts have been simplified as far as possible
without losing the original sense. A calculator is not essential but it is a
labour saving tool, producing in seconds, that which could take many minutes
or even hours.
That which follows is written on the assumption of nil knowledge on the part of the reader. Accordingly, it is necessary to restate some fundamental concepts. The explanation I have given for some of those concepts is not necessarily scientifically correct, because in the final analysis, a concept is no more than a frame, upon which we hang our understanding. Therefore, I feel that it is of more importance, to be almost right and understandable, than it is to be scientifically pure and totally incomprehensible. Because in the practice one will find many things, which taken scientifically are just not so.
The Theory of Matter 4.2
We do not know what matter really
is. We do not know what a Proton
is made of.
We do not know what an Electron is made of, And
the words only serve to cloak our ignorance.
Professor Louis C. Kervran
Biological Transmutations.
All substances are composed of small particles called molecules, which are composed of even smaller particles called atoms, which in their turn are composed of even smaller particles, ad-infinitum. The essential point is, that the major difference between those particles, is that the molecule is the smallest particle of a substance that can exist and maintain all the chemical properties of that substance and which allows us to say, �this is air� and �that is water�.
The Atom 4.3
The structure of an atom has been
visualised as resembling a miniature solar system, ie, a central sun with a
complement of planets in spaced orbits around it. The concept is elegant and
has allowed science to deduce many explanations of the myriad natural
phenomena that surround us, but more important, we are able to reproduce
phenomena on demand, the result of which, is the technical wonderland of
which we know, and take for granted.
The atom consists of three major types of particles;
1. The proton, which is positively charged and forms a part of the nucleus or sun.
2. The electron, which has negative charge and which orbits the nucleus in the same way that a planet orbits the sun.
3. The neutron, which is said to be neutral, ie, holding no charge. It shares the nucleus with the proton, so it may be understood as a gravity that prevents the disintegration of the nucleus. The nucleus also has spin.
Figure 4.3A
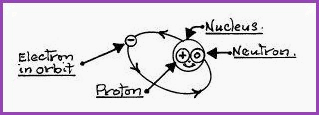
It is believed that there are other sub-atomic particles, but for our purpose they have no relevance. An atom has the same number of electrons as protons therefore its electrical potential is zero. The orbiting electrons are of two types;
1. Bound electrons which occupy the inner orbits.
2. Free electrons which occupy the outer orbits or shells.
If external attraction or pressure is applied to an atom, then one or more of the free electrons can be moved to another atom and a chemical reaction occurs in which the two atoms combine to form a compound.
Therefore when an atom undergoes a chemical change it does so in the form of charged particles, which are called �ions�, which are said to be of two types, i.e. negative ion and positive ion.
A negative, is an atom with an extra electron, and a positive, is an atom which is minus an electron. Therefore on the same principle as a magnet, like charged ions repel and unlike charged atoms attract. The nucleus of the atom does not appear to play any part in this type chemical change.
The Heart of the Matter 4.4
Matter may exist in four basic states;
1. As a solid, for example, an ice cube.
2. As a liquid, e.g. water.
3. In a gaseous state, e.g. steam.
4. In the radiant state, e.g. the photon stream from the sun.
Matter is anything that has mass and volume, irrespective of its state. For the purpose of clarity, matter may be placed into three categories;
1. Elements. 2. Compounds. 3. Mixtures.
Elements 4.5
An element is the basic stuff of the
physical universe. An element is composed of 2 or more atoms of the same
type, which are called molecules, which are chemically bonded. An
element cannot be further divided by chemical means.
Elements differ from each other by the number of sub-atomic particles contained within their respective atoms. Scientifically, elements are differentiated from each other by their atomic weight. The atomic weight is the sum of the mass of protons and neutrons found within the atomic nucleus. The atomic number of an element is the number of protons in its nucleus.
Compounds 4.6
Compounds are comprised of 2 or more
elements of different types, or two or more compounds of different types
which are chemically bonded. When bonding takes place a new substance is
formed. The new substance will have different properties than those of the
elements that formed it. For example, H20. Two atoms of hydrogen
gas bond with one atom of oxygen gas to form water.
Figure 4.6A
Mixtures 4.8
A mixture is two or more elements, or
elements and compounds, which are not chemically bonded and can be separated
by physical rather than chemical means. (See Section 5-9). The element or
compounds that comprise a mixture retain their own properties and may act
independently or in conjunction; for example, the mixture of air and water
vapor having a high humidity.
There are three kinds of mixtures;
1. Two or more elements that are not chemically bonded. 2. Two or more compounds which are not bonded. 3. Elements and compounds mixed but not bonded.
The three types of mixtures may be further classified into two types,
1. Homogenous - having uniform properties.
2. Heterogenous - properties are not uniform.
Summary Fig 4.8A
Physical and Chemical Processes 4.9
Many methods have been devised to
physically separate mixtures into pure components. Most are based on the
differing properties of matter and are reversible processes; e.g. a magnet
could be used to separate iron filings from sand, or a sieve could be used
to separate sand from flour or chaff from grain.
Distillation is used to separate the impurities from water. Fractional distillation is used by the Petroleum Industry in the processing of crude oil. Advantage is taken of the differing boiling points of the various fractions of the oil, thus providing a variety of products which are in everyday use, e.g. petroleum spirit, kerosene and natural gas, such as butane and propane.
When a mixture has been separated into individual components, the resulting parts are classified as pure substances that have uniform properties and cannot be further divided by physical means. Pure substances may be either elements or compounds. A compound can only be separated (decomposed) by a chemical reaction. As a scientific process the separation can only be achieved with the aid of high temperatures or strong acids, or electrolysis and is not reversible, e.g., burnt toast is a non reversible chemical change initiated by a domestic process.
Cane sugar provides a further example; it can only be decomposed in a kitchen or laboratory, by burning it, the product is caramel. Nature can decompose sugar at low temperatures by the biological process of fermentation to produce alcohol. However if sugar is dissolved (entered into solution), in warm water it may be recovered, intact, by the evaporation of the water, in the same way that salt is recovered from sea water.
So to summarize, a chemical change involves a change of substance which has properties different from those of the original substance. For the purpose of dehydration, the emphasis is on physical change, which involves a change of state, in which the properties of the substance remain the same and the process is reversible.
Fig 4.9A
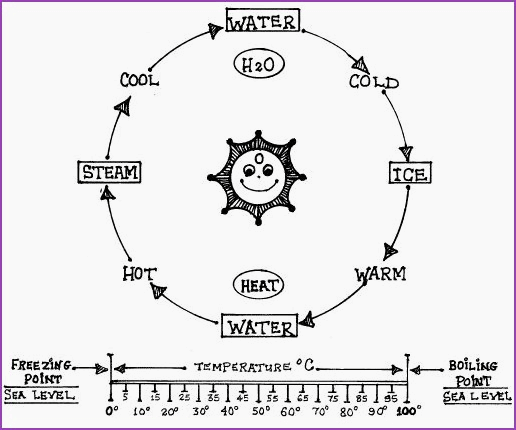
Atoms � Molecules � Elements and Energy
4.10
A glance at the Periodic Table of the
elements (Table 4.10A), will show that the elements are arranged in seven
rows.
If we stay with the flying particle, or solar system model of the atom, then each row or period, will represent a planetary, or electron orbital track around the sun or nucleus; thus depending on the element it is possible to have up to seven orbital tracks around the nucleus. There is an upper limit to the number of electrons that can be contained in any one orbit. For example; the first period, or orbit, can contain 1 or 2 electrons. The second period, or orbit, can contain up to 8 electrons, whilst the 6th period, or 7th orbit, could hold up to 32 electrons. Therefore, it may be seen that elements differ from each other, not only by their period, but also in the number of particles that they contain.
Compare Figure 4.10A with Table 4.10A
Fig 4.10A
The number following the name of the element is its atomic number.
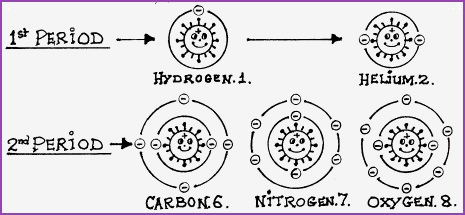
If we now move away from the flying particle model and visualise the electron orbits as energy levels, or energy shells, then it will be readily understood that the elements may also be graded on an ascending scale of energy content. The energy content of a molecule manifests as a vibration which is known as its frequency.
The. The formula weights for air and water are different and need differing amounts of heat to raise their respective temperatures to the same level.
It will be seen from the Periodic Table, that in addition to the 7 periodic rows, that there are 18 columns. The frequency of any substance is the number of cycles or vibrations performed in 1 second. Therefore, the higher the atomic number, the higher the frequency. If we add energy to a substance in the form of heat its rate of molecular vibration will increase in proportion to the amount of heat added. Likewise, if we remove energy the vibration will decrease in proportion to the energy removed. It then follows that the same amount of heat added to different substances will manifest differing temperatures, this concept will be expanded in following sections.
The sum of the atomic weights of the atoms in a chemical formula, (eg.H2O) equals the weight of the compound formula.
The columns represents chemically related substances; the idea will be grasped if columns 11, 12 and 18 are examined.
The key to the chemical symbols are found in Table 4.10B. The full name of the elements are in alphabetical order.
Periodic Table 4.10A
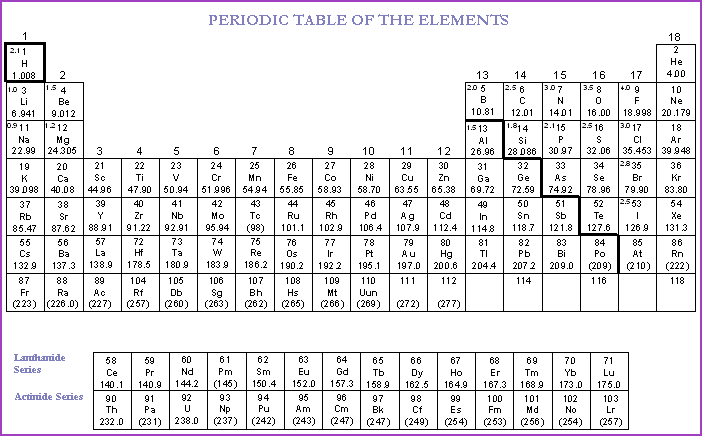
N.B. The periodic table is in a continual state of flux as new elements are added to the Actinide series. These new elements are not found in Nature. For our purpose they are irrelevant.
Alphabetical list of the elements. Figure 4.10A
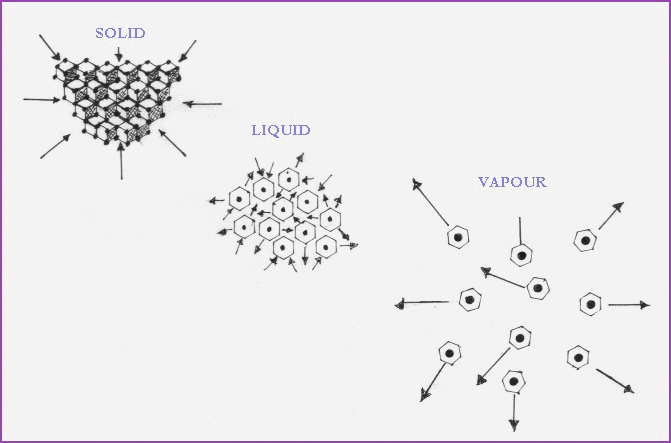
The 3 Physical States of Matter 4.11
Figure 4.9A illustrates the 3 physical
states of water. From it we may infer that heat or lack of it, may be used
to alter the physical state of a substance.
Figure 4.11A
Solids 4.12
A solid has a definite shape and a fixed
volume, it is difficult to compress, it expands when heated and contracts
when cooled. For our purpose the effects of pressure upon a solid have no
relevance ,therefore, it is ignored. A solid is different from a liquid, in
that the molecules arrange themselves in a lattice work of the greatest
possible cohesion. In other words they cannot move independently of each
other, and their movement is limited to vibration.
When a solid is heated, then its molecules commence to vibrate at a greater rate. If we continue to add heat the vibratory pitch reaches a point where the individual molecules start to break away from the lattice and start to move independently. We can observe when that happens because the solid starts to melt. The freed up molecules take the extra energy with them so one must continue to add heat to the solid to bring about total meltdown.
Liquids 4.13
The shape of a liquid is determined by its
container. It has a fixed volume and is not easily compressed. Like a solid,
liquids will expand when heated and contract when cooled. The molecules of a
liquid are in constant motion. The range of movement is greater than that of
a solid. The motion is limited to erratic movements with the molecules in
constant collisions, somewhat like a pinball machine.
As a result of the constant collisions, some of the molecules at the surface of the liquid are traveling at a greater speed than those at the interior. If the acceleration of the molecule is great enough to break its bonding, it will launch itself into the surrounding air. The more heat added to the liquid, the faster the molecular motion, with ever growing numbers of molecules escaping to become vapour. The temperature at which that change of state occurs is called the boiling point. The evaporation of water molecules occurs at all temperatures above freezing.
Vapours 4.14
Vapour, like a gas, has no shape and it
will fill or disperse in any available space. It can be compressed, and like
a solid or liquid, it will expand when heated and contract when cooled. The
vapour molecules have broken free of the attraction that bound them together
as a liquid, consequently, and in relation to their size, the space between
each molecule is of great magnitude, e.g. the volume of a 1 kg of water
vapour at atmospheric pressure (sea level), is 1600 times that of 1 kg of
water. The movement of vapour molecules is quite free, and is only limited
by a vessel that contains them, or by collisions with each other.
The difference between a vapour and a gas is partly in definition and partly of behaviour. Vapour is called a vapour when it is at a temperature at which it can exist as a liquid, providing that there is sufficient pressure upon it. If a vapour is hotter than the temperature at which it can exist as a liquid, irrespective of the pressure upon it, then it is a gas. A gas will obey well established laws relating to its change of volume with varying temperatures and pressures, whereas a vapour will only obey the gas laws within limits.
In relation to dehydration, we use atmospheric gases to remove herb water vapour, so it is important to remember that a distinction exists.
Library
Pharmageddon
Herbal Block Index
![]()