OLIVE OIL � OLEUM OLIVAE
United States
Dispensatory 1926
Compiled
by Ivor
Hughes
OLEUM
OLIVAE.
U. S.
, Br. OLIVE OIL
OL OIiv.
The fixed oil obtained from the ripe fruit of Olea
europaea Linne (Fam. Oleaceae)." U. S. "
Olive Oil is the oil expressed from the ripe fruit of Olea
europaea, Linn., and refined."
Br.
Sweet
Oil; Huile d'Olive, Fr. Cod.; Olivarum Oleum, P. G; Olivenỏl,
G.; Olio di olive. It.; Aceite
de olivas, Sp.
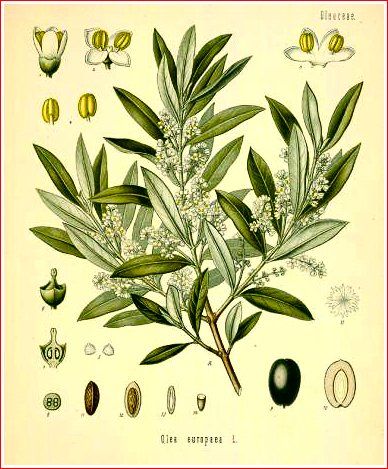
Olea europaea L. is one of the leading fruit trees
of the world. It is usually from fifteen to twenty-five feet in height,
though sometimes much larger, especially in
Greece
and the
Levant
.
It has a solid, erect, unequal stem, with numerous straight branches,
covered with a grayish bark. The leaves are evergreen, firm, oblong or
lanceolate, entire, two or three inches in length, smooth and of a dark
green color on their upper surface, whitish and almost silvery beneath.
The flowers are small, yellowish white, and disposed in opposite axillary
clusters, about half as long as the leaves, and accompanied with small,
obtuse, hoary bracts. The fruit, or olive, is a smooth, oblong or oval
drupe, greenish at first, but of a deep violet color when ripe, with a
fleshy, mesocarp and a very hard endocarp. Clusters of
not less than thirty flowers yield only two or three ripe olives.
The olive tree, which is probably native to western
Asia
or the eastern Mediterranean district, flourishes at present in all the
countries bordering on the
Mediterranean
,
and has been cultivated from time immemorial in
Spain
,
the south of
France
,
and
Italy
.
It begins to bear fruit after the second year, is in full bearing at six
years, and continues to flourish for a century. There are forty varieties,
distinguished by the form of the leaves and the shape, color, and size of
the fruit. The variety longifolia
of Willdenow is said to be chiefly cultivated
in
Italy
and the south of
France
,
and the latifolia in
Spain
.
The latter bears much larger fruit than the former, but the oil is less
esteemed. The olive is largely cultivated in the north of
Africa
,
especially in the vicinity of
Tunis
,
southern
California
and other warm regions.
Olives are cultivated especially in
Spain
in the district of Cadiz. Two classes are produced, the one known as the "
Queen olive," a large olive, which is mostly exported to the
United States
;
the other the Manzanillo, or small olive, which is chiefly consumed in
Spain
,
South
America
and
Cuba
.
Pickled olives, made by soaking green olives first in dilute solution of
sodium hydroxide and then in salt water, are largely used as an article of
food; the ordinary green olive of commerce has been picked before
ripening. The ripe olive, which, in the
United States
,
is chiefly obtained from
California
,
is dark-purple, often almost black, very different in taste from the
ordinary unripe pickled fruit, and is said to contain about 50 per cent,
of olive oil, so that it affords an excellent method of administering:
fat.
There have been reported several epidemics of a peculiar form of
food poisoning, known as botulism, from the eating of ripe olives which
had been improperly preserved. This disease is caused by the Bacillus botulinus, or the toxins produced by it. It follows the use
not only of olives, but of many other foods which have not been properly
sterilized. The most prominent symptoms, which usually begin a few hours
after the ingestion of the peccant food, are: Nausea, vomiting and
diarrhea, with abdominal cramps, followed by constipation: there are
severe nervous disturbances, especially of the visual apparatus�dilatation
of the pupils, diplopia, and ptosis�loss of voice, dryness of the throat
and great thirst, followed by exhaustion and, in many eases, death,
sometimes preceded by delirium.
The
leaves and bark of the olive tree have an acrid and bitter taste, and have been
employed as substitutes for cinchona, though with no great success. In hot
countries, a substance resembling the gum-resins exudes spontaneously from
the bark. It was thought by the ancients to possess useful medicinal
properties, but is not now employed. Analyzed by Pelletier, it was found
to contain resin, a little benzoic acid, and a peculiar principle
analogous to gum, which has been named olivile.
But the fruit is by far the most useful product. In the unripe state the
fruit is hard and insupportably acrid, but when macerated in water or an
alkaline solution, and afterwards introduced into a solution of common
salt it loses these properties, and becomes a pleasant and highly esteemed
article of diet. Mannite has been found in all parts of the tree while in
vital activity, as in the green leaves and unripe fruit, but cannot be
detected in the yellow fallen leaves or in the perfectly ripe fruit.
The
pericarp, or fleshy part of the ripe olive, abounds in a fixed oil, from 10
to 20 per cent., which constitutes its greatest value, and for which the
tree is chiefly cultivated in southern
Europe
.
The olives ripen from November to March, and the oil is obtained by first
bruising them in a mill and then submitting them to pressure. The product
varies much, according to the state of the fruit and the circumstances of
the process. The best, called virgin
oil, is obtained from the fruit picked before perfect maturity, and
immediately pressed. It is distinguished by its greenish hue. The common
oil used for culinary purposes and in the manufacture of soaps is procured
from very ripe olives, or from the pulp of those which have yielded the
virgin oil. In the latter case the pulp is thrown into boiling water, and
the oil removed as it rises. An inferior kind, employed in the arts,
especially in the preparation of the coarser soaps, plasters, unguents,
etc., is afforded by fruit which has been thrown into heaps, and allowed
to ferment for several days, or by the marc left after the expression of
the finer kinds of oil, broken up, allowed to ferment, and again
introduced into the press.
Description
and Physical Properties. � A pale yellow,
or light greenish-yellow, oily liquid, having a slight, peculiar odor and
taste, with a faintly acrid after-taste. Olive Oil is slightly soluble in
alcohol, but is miscible with ether, chloroform, and carbon disulphide.
"Specific gravity: 0.910 to 0.915 at 25� C. When cooled to from 10�
to 8" C., the Oil becomes somewhat cloudy from the separation of
crystalline particles, and at 0� C. it usually forms a whitish granular
mass. Mix 6 cc. of the Oil in a test tube with 5 cc. of a mixture of equal
volumes of amyl alcohol and carbon disulphide, the latter containing 1 per
cent, of precipitated sulphur in solution, and immerse the test tube to
one-third of its depth in boiling, saturated aqueous solution of sodium
chloride: no reddish color develops in fifteen minutes (cottonseed oil).
Mix 2 cc. of the Oil with 1 cc. of hydrochloric acid containing 1 per
cent, of sucrose, shake the mixture for half a minute, and allow it to
stand for five minutes: on adding 3 cc. of distilled water to the mixture
and shaking it, the acid layer shows no pink color (sesame oil). Saponify
10 Gm. of Olive Oil by heating it under a reflux condenser with a solution
of 4 Gm. of potassium hydroxide in 80 cc. of alcohol for one hour.
Neutralize exactly with diluted acetic acid, using phenolphthalein T.S. as
indicator, and wash into 120 cc. of boiling lead acetate T.S. Boil the
mixture for one minute, and cool by immersing the flask in cold water,
occasionally rotating the contents to cause the precipitate to adhere to
the sides of the flask. Decant the liquid, wash the precipitate with cold
water to remove excess of lead acetate, then wash with 90 per cent,
alcohol (by volume). Add 100 cc. of ether, cork well, and allow to
stand until the precipitate is disintegrated. Connect with a reflux
condenser, boil for five minutes, cool to about 15� C. and allow to
stand over night. Filter, and thoroughly wash the precipitate of
lead soaps with ether. Wash the precipitate into a 500 cc. separator by
means of a jet of ether, alternating with diluted hydrochloric acid at the
end, (if a little of the precipitate adheres to the filter paper. Add
enough diluted hydrochloric acid to make the total acid layer about 100
cc., and enough ether to make the total ether layer about 100 cc. and
shake vigorously for several minutes. Allow the layers to separate, draw
off the acid layer, and wash the ether once by shaking with 50 cc. of
diluted hydrochloric acid and then with several portions of water until
the water washings are no longer acid to methyl orange T.S. Transfer the
ether solution to a dry flask, evaporate the ether, add a little
dehydrated alcohol and evaporate on a steam bath. Dissolve the dry fatty
acids by warming with 50 cc. of 90 per cent, alcohol (by volume), slowly
cool the solution to 15� C., shaking frequently to aid crystallization,
and allow the mixture to stand at 15� C. for thirty minutes. No crystals
separate (peanut oil). Saponification value: not less than 190 and not
more than 195. Iodine value: not less than 79 and not more than 90.
Preserve
in well-closed containers, in a cool place." U. S. "
Pale yellow or greenish yellow. Faint but not rancid odor;
taste bland. Frequently assumes a pasty consistence when maintained for
some time at a temperature of 10� C., and at a lower temperature may
become a soft granular mass. Specific gravity 0.915 to 0.918;
Saponification value 188 to 197; iodine value 79 to 87; acid value not
more than 6.0; refractive index at 40� C. 1.4605 to 1.4635. A mixture of
2 millilitres of the Oil with 1 millilitre of amylic alcohol and 1
millilitre of a solution (1 in 100)" of precipitated sulphur in
carbon disulphide', placed in a test-tube immersed in boiling water, does
not assume a red color within thirty minutes (absence of cottonseed oil).
When a mixture of 2 millilitres of the Oil and 1 millilitre of
hydrochloric acid containing 1 per cent, of refined sugar is shaken for
half a minute, and allowed to stand for five minutes, the acid layer does
not become pink (absence of sesame oil). When 1 millilitre of the Oil and
15 millilitres of N/l alcoholic solution of potassium hydroxide are boiled
for twenty minutes in a flask provided with a reflux condenser, set aside
for twenty-four hours at a temperature not exceeding 15.5� C., and
afterwards heated on a water-bath for three minutes, the solution does not
deposit crystals on standing for twenty-four hours further (absence of
arachis oil)."
Br.
The concrete portion, about 28 per cent of the oil, which separates at a
freezing temperature, consists chiefly of the glyceride of palmitic acid,
together with a smaller amount of glyceride of arachidic acid (stearic
acid is stated by Hehner and Mitchell to be
absent entirely); the liquid portion is essentially olein, with a small
amount (seven parts for ninety-three of olein) of the glyceride of the
less saturated linoleic acid. According to Braconnot, the oil contains 72
per cent, of olein and 28 of palmitic. A small quantity of phytosterin has
also been found in the oil. Olive oil is solidified by nitrous acid and
mercuric nitrate, and converted into elaidin. (See under Olea Fixa.) The
greenish color is owing to the presence of a trace of chlorophyll, and a
trace of cholesterin is also extracted by repeated agitation with glacial
acetic acid. For discussion of the spectra of olive oil, see P. J., 3d
series, vii, 22, 110.
Olive oil, when exposed to the air, is prone to become rancid, acquiring a
disagreeable odor, a sharp taste, and a thicker consistence; it also loses
its color, and the change is promoted by heating it. It is frequently
adulterated with the cheaper fixed oils, especially with cottonseed oil,
peanut oil, sesame oil and poppy seed oil. Of these, sesame is easily
recognized; for peanut oil there is as yet no reliable color reaction, and
while Bechi's reaction may, under certain conditions, answer
well for the detection of cottonseed oil, this, under others, has also
proven unreliable. (Ph. Ztg., 1904, 104.)
According to Kreis and Grob
(S. W. P., 1901, 88), Billier's test is very sensitive. It is carried out
by shaking the suspected oil with a solution of resorcinol in benzene and
afterwards with nitric acid. Olive oil under these circumstances remains
unchanged in color, cottonseed and nut oil become red violet and maw seed
oil is turned brown red. (See also official test for peanut oil.)
Bechi�s
test for ascertaining the admixture of cottonseed oil with olive oil
has received the approval of the Commission of Florence after most
exhaustive experiments, and is as follows: One grain (0.065 Gm.) of silver
nitrate is dissolved in fifteen minims (0.9 cc.) of water, and six and a
half fluidounces (195 cc.) of alcohol are added. Two fluidounces (60 cc.)
of ether may be added to render the solution more easily miscible with the
oil, but it is not absolutely necessary. A solution of eighty-five parts
of amyl alcohol and fifteen parts of rape seed oil is prepared. These
reagents should not be kept on hand any length of time. To ten cc. of the
oil to be examined one cc. of the solution of silver nitrate is added, and
then from eight to ten cc. of the amyl alcohol reagent; the mixture is
agitated strongly, and heated on a water bath for five or ten minutes. In
the case of pure oils the color is unaltered by the addition of the
reagents; if cottonseed oil be present, a brownish color or turbidity,
varying from a light brown to a deep maroon or black (according to the
extent of the adulteration), will be produced. (A. J.
P., 1887.)
Milliau's
test is a modification of the foregoing. If the fatty acids instead
of the oil itself are treated with the same reagents, the same reduction
of the silver salts occurs and the same brown color as in the Bechi
test.
Halphen's
test with a mixture of amyl alcohol and carbon disulphide containing
about 1 per cent, of sulphur in solution is now regarded as the most
distinctive for cottonseed oil. For details see the official test. Lewkowitsch
(Chemical Analysis of Oils, Fats, and Waxes, 2d ed., 1898, 462) says that
the color reactions proposed by various authors are altogether unreliable
and yield no definite results, with the exception of the color test
(Baudoin's test, see Oleum Sesami) for sesame oil and perhaps Bechi's and
Milliau's or Halphen's for cottonseed oil. Bulletin 77 (1905), of the
Bureau of Chemistry, United States Department of Agriculture, showed that
most of the spurious and adulterated olive oil in the United States was of
domestic origin and that the oil as imported in original packages was
mostly genuine. This has been verified by numerous other investigators
during recent years. Since the passage of the Food and Drugs Act in 1906
it is rare to find a labelled container of olive oil adulterated, although
much that is sold in bulk is still found to be spurious. In several states
it has been judicially decided that the synonyms "sweet oil" and
"salad oil" when unqualified mean olive oil. A low grade of
olive oil used for soap making and other technical purposes is imported at
a less rate duty than is collected on edible olive oil. The frequent use
of such low grade oil for table purposes by those who are not particular
about quality and flavor led to the requirement on the Part of the U. S.
Treasury Department that such "technical oil" must be denatured
for admission. There are three alternative and optional methods of
denaturing. They consist in adding to the oil a small portion of oleoresin
of capsicum, of oil of rosemary or of kerosene. Arachis oil, which is
produced in large quantities in
Marseilles
,
has been found in numerous samples of imported olive oil.
According to Tambon, the present-day adulterations do not consist in the
addition of a single cheaper oil, but of
ingeniously prepared mixtures of different oils in such proportions to
each other that on analysis it becomes difficult or impossible to
distinguish the adulterated from the genuine oil by the chemical and
physical constants accepted for the latter.
Uses.� Olive oil is nutritious and mildly laxative and is often used in
milder cases of chronic constipation, especially when associated with
malnutrition. (See J. A. M. A,, 1919, Ixxiii,
1441.) In the form of enema it is often a useful remedy in fecal
impaction. It was formerly largely used in the treatment of gall stones
with the idea that it stimulated the secretion of bile. The concretions
which were passed by these patients, however, were shown to be masses of
hardened soap and it is extremely questionable whether the oil has any
real value in this disease. Externally it is useful to soften and relax
the skin and to protect it against the action of the air. The most
extensive use of olive oil is in pharmacy as a constituent of liniments,
ointments, cerates, and plasters.
Dose,
as a laxative, from one-half to two fluidounces (15-60 cc.).
Off. Prep.� Linimentum
Ammoniae, "Br.; Linimentum Calcis, Br.; Linimentum Camphorae, Br.;
Oleum Phenolatum, N. F.; Unguentum Fuscum, N. F.
See
also � Fixed Oils, Martindales 24th
Did
you find what you were looking for? If not please use the site search box
at the top right hand of the page or else return to the site library.


![]()