
B.P. 1958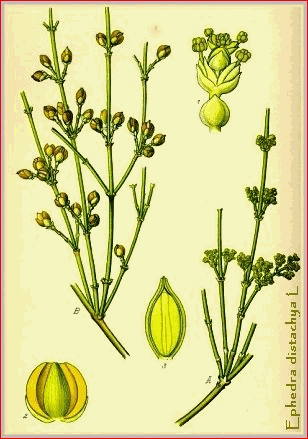 EPHEDRINE
� EPHEDRA.
EPHEDRINE
� EPHEDRA.
BRITISH PHARMOCOPOEIA 1958
U.S.D. 1926
EXTRA PHARMACOPOEIA - MARTINDALES 24th (Br)
Compiled and Edited by Ivor Hughes
Ephedrine is the hemihydrate of (-)-2-methylamino-l-phenyl-propanol, an
alkaloid obtained from Ephedra sinica Stapf, Ephedra
equisetina Bunge, and other species of Ephedra, or prepared
by synthesis. It contains not less than 94-0 per cent and not more than 95-0
per cent of C10H15ON.
Description. Colourless, non-deliquescent, non-efflorescent,
hexagonal, prismatic crystals; odourless, or with a slight, unpleasant
odour; taste, bitter.
Solubility. Soluble, at 20�, in 36 parts of water, in less than 1
part of alcohol (95 per cent), in solvent ether, and in chloroform with
separation of water. Soluble, at 15-5�, in 20 parts of glycerol; soluble in
100 parts of liquid paraffin, with separation of water.
Identification.
B. Dissolve 0-2 g. in 30 ml. of chloroform and set aside for twelve hours, allowing the chloroform to evaporate at room temperature. Wash the crystals of ephedrine hydrochloride which separate with 10 ml., 5 ml., and 5 ml. of chloroform and dry at 105�; the crystals have a melting-point of about 218�, page 822.
C. The crystals from test B yield the reactions characteristic of chlorides. Alkalinity. A solution is strongly alkaline to litmus solution.
EPHEDRINE HYDROCHLORIDE
Specific rotation. Dissolve an accurately weighed quantity, calculated from the Assay to be equivalent to 2-05 g. of anhydrous ephedrine, in 15 ml. of dilute hydrochloric acid and dilute to 50 ml. with water. The specific rotation of this solution is � 33� to � 35-5�, calculated with reference to the ephedrine hydrochloride produced from the base.
Chloride. Dissolve 0-10 g. in 1 ml. of water and 1 ml. of dilute nitric acid and add 0-1 ml. of silver nitrate solution; no turbidity is produced.
Oxalate. Dissolve 0-50 g. in 5 ml. of water and 1 ml. of dilute hydrochloric acid and add a slight excess of dilute ammonia solution and 0-5 ml. of calcium chloride solution; no opalescence is produced within ten minutes.
Sulphate. Dissolve 0-10 g. in 1 ml. of water and 1 ml. of dilute hydrochloric acid and add 0-5 ml. of barium chloride solution; no turbidity is produced within ten minutes.
Sulphated ash. Not more than 0-1 per cent.
Assay. Dissolve about 0-5 g., accurately weighed, in 5 ml. of alcohol (95 per cent), add 35 ml. of N/10 hydrochloric acid, and titrate with N/10 sodium hydroxide, using methyl red solution as indicator. Each ml. of N/10 hydrochloric acid is equivalent to 0-01652 g. of C10H15ON.
United States Dispensatory 1926
Ephedra. Ephedra vulgaris Rich. (Fam. Gnetaceoe.) �This gymnospermous plant is a straggling shrub with long-jointed and fluted green stems and scale-like opposite leaves forming at each joint a two-toothed sheath. It grows in Eastern Europe and Western Asia. It has been used as a medicine by the Chinese for thousands of years, being included in the collection made by the Emperor Shen-Nung about 1500 B.C.; in China it is known as Ma huang. For pharmacognostic description see Chen (J. A. Ph. A., 1925, xiv, 189).
In 1887 Nagai isolated from this plant an alkaloid ephedrine, C10H15ON. Subsequently Merck found in the European variety an alkaloid which he called pseudo-ephedrine, apparently isomeric with that from the Asiatic variety. Ephedrine occurs as colorless crystals melting at 214� C., while pseudo-ephedrine forms yellowish crystals and melts at 175� C. Spehr (A. J. P., 1892, 234) found in the E. Monostachya L. an alkaloid, C13H19ON, which was physiologically inert.
Ephedrine has been studied by Miura, Amatsu and Kubota and by Chen and Schmidt (J. P. Ex. T., 1924, xxiv, p. 339) with concordant results. Chemically it is closely related to epinephrin, differing only in that two hydroxyl groups of the latter have been replaced by hydrogen atoms. In physiological action also it resembles epinephrin. It causes a marked rise in the blood pressure with an increase of both the rapidity and excursion of the heartbeat. The rise of blood pressure is also due in part to vasomotor stimulation. It causes dilatation of the pupil, whether injected into the blood stream or applied locally, without loss of the power of accommodation. It relaxes the intestinal muscle and apparently also the 'bronchial. All these effects seem to be due to stimulation of the sympathetic nervous system.
Chen and Schmidt find that it differs from epinephrin chiefly in the persistence of effect and in the fact that it is active when taken by the mouth. A dose of 60 milligram�s in a human being produced a rise of blood pressure of 20 millimeters that lasted for two hours. They have used it clinically as a circulatory stimulant in doses of from 40 to 60 milligram�s (one-half to one grain), either hypodermically or by mouth with good effects. They also record a case of Addison's disease in which the drug produced a marked alleviation of symptoms.
An alkaloid has also been discovered in Ephedra distachya L., a shrub whose branches and root are used in Siberia as a remedy in gout and syphilis. According to Robert, this alkaloid is essentially different from ephedrine, in not being mydriatic or poisonous. E. monostachya contains a crystalline base melting at 112" C. and having the formula C13H19ON. This latter, as stated by Robert, is not mydriatic or poisonous. See paper by E. Schmidt, A. Pharm., 1912, ccl, 154, 161.
Ephedra nevadensis S. Wats, and E. antisyphilitica C. A. Meyer, which grow abundantly in the extreme Western United States, are used under the names of caynote, canutilo, whorehouse tea as a remedy in gonorrhea. Loew thinks that their virtues reside in a peculiar tannin.
Dose,
of fluid-extract, from one to two fluidrachms (3.75-7.5 cc.). In Texas E, trifurca Torr. is similarly employed.THE EXTRA PHARMACOPOEIA � MARTINDALES 24th (Br)
Ephedra (B.P.C.). Ma-huang.
Foreign Pharmacopeias: In Chin,, Fr., Ind., and Jap.
Ephedra consists of the dried young branches of Ephedra sinica, E.
equisetina and E. gerardiana (including E. nebrodensis)
(Ephedraceas), containing not less than 1-25% of alkaloids, calculated as
ephedrine. Protect from light and moisture.
Uses. The action of Ephedra is due to the presence of ephedrine and
pseudoephedrine. It is used mainly as a source of the alkaloids.
Liquid Extract of Ephedra (Ind. P.} Ext. Ephed. Liq. An
alcoholic extract containing 2% w/v of alkaloids.
Tincture of Ephedra (Ind. P.). Tinct. Ephed. Liquid extract of ephedra 25 g., alcohol (40%) to 100 ml. It contains 0-5% w/v of alkaloids.
Dose: 6 to 8 ml. (90 to 120 minims).
THE EXTRA PHARMACOPOEIA PROPRIETARY PREPARATION CONTAINING EPHEDRA
Phedros (Merck Sharp & Dohme). A cough syrup containing in
each fl. oz. Ephedra liquid extract 40 m., Squill liquid extract 3 m.,
Ipecacuanha liquid extract 2.8 m., ammonium chloride 8 gr., chloroform 2 m.,
and Syrup of Tolu 72 m., with dehydrated alcohol 6% v/v.
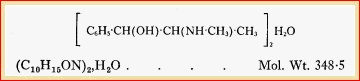
Ephedrine (B.P.). Ephed. (�)-2-Methylamino-l-phenylpropan-l-ol
hemihydrate. C10H15ON, � H2O = 174-2.
Foreign Pharmacopoeias: In Belg., Dan., Egyp., Fr., Ind., Mex., and Swed. Also in U.S.N.F.
Fr., Mex., and U.S.N.F. specify anhydrous or hemihydrate. Span, specifies anhydrous only.
An alkaloid obtained from species of Ephedra, or prepared synthetically. Colourless, odourless, non-deliquescent, non-efflorescent, laevorotatory crystals with a bitter taste; a faint aromatic odour may develop on storage. M.p. 40� to 41�. Solutions in water are alkaline to litmus and decompose in air and light; solutions in oil may develop an alliaceous odour.
Soluble at 20�, 1 in 36 of water, 1 in less than 1 of alcohol, in ether, and in chloroform with turbidity due to separation of water; soluble, at 15-5�, 1 in 20 of glycerin, 1 in 25 of olive oil, and 1 in 100 of liquid paraffin with separation of water; the anhydrous alkaloid forms clear solutions in liquid paraffin. Incompatible with iodine, silver salts, and tannic acid. Protect from light in a cool place.
Toxic Effects. When given in sufficiently large doses ephedrine gives rise in most patients to certain minor reactions, such as giddiness, headache, nausea, vomiting, sweating, palpitations, difficulty in micturition, tremors, anxiety, restlessness and insomnia; some patients may exhibit these symptoms even with the usual therapeutic dosage. On the other hand single doses of as much as 400 mg. (6� grains) have been given without causing serious toxic effects. In hypersensitive patients local application of the drug may cause a contact dermatitis.
Antidotes. The stimulant effect of ephedrine on the central nervous system may be counteracted by administration of barbiturates.
Contra-indications. The use of ephedrine is contra-indicated in the presence of coronary thrombosis. It should be used with caution in patients with organic heart disease, cardiac decompensation, hyperthyroidism, hypertension, and angina pectoris, and in patients receiving digitalis.
Uses. Ephedrine is a sympathomimetic amine which is effective when taken by mouth. It has a more prolonged though less potent action than adrenaline. In therapeutic doses it produces peripheral vasoconstriction and raises the blood pressure; this effect is slower and more sustained than after adrenaline. Tachycardia may occur but is less frequent than with adrenaline. Ephedrine also causes bronchodilatation, reduces intestinal tone and motility, relaxes the bladder wall while contracting the sphincter muscle, and contracts the uterus. It has a stimulant action on the cerebrum and the respiratory centre. It dilates the pupil but does not affect the light reflexes. After using ephedrine for a short while tachyphylaxis may develop and a much larger dose may be required to give the same effect as before; interruption of treatment for a few days will often restore sensitivity. Prolonged administration does not give rise to cumulative effects nor to addiction.**
** Editors Note:
This statement has proven to be hideously wrong, just as it has been in many other cases, e.g. Heroin and Cocaine. The isolated alkaloids and also the synthetics are extremely dangerous. Natures safety barriers have been discarded.Ephedrine hydrochloride or sulphate is given in doses of 30 to 60 mg. (� to 1 grain) by subcutaneous or intramuscular injection to maintain or restore blood pressure during spinal anaesthesia. The dose is generally injected 30 to 45 minutes before giving the anaesthetic. For rapid effect 24 mg. (⅜ grain) may be given intravenously. Ephedrine is of little value in hypotensive crises due to shock, circulatory collapse, or hemorrhage. For chronic hypotensive states and postural hypotension 16 to 60 mg. (� to 1 grain) of ephedrine salts may be given by mouth every 2 to 4 hours, the first dose each day being taken one hour before rising. Ephedrine may also be of benefit in the symptomatic treatment of complete heart block with syncope. The initial oral dose of 16 mg. (� grain) should be increased gradually to 48 mg. (� grain) or more, three or four times daily. It must not be used if the Stokes-Adams syndrome is due to ventricular fibrillation.
In the treatment of narcolepsy and catalepsy the usual dose of ephedrine is 8 to 60 mg. (⅛ to 1 grain) three times a day by mouth. It has been used in the treatment of myasthenia gravis, usually with neostigmine. In enuresis, ephedrine is of value partly because of its effect on the bladder and partly because of its central stimulant action. Usually 60 to 120 mg. (1 to 2 grains) is given by mouth at bedtime, increased, if necessary, by 30-mg. (� -grain) increments to 300 mg. (5 grains); in smaller doses it may be given to children. In elderly men it may cause urinary retention.
Ephedrine hydrochloride or sulphate, in doses of 16 to 60 mg. (� to 1 grain) by mouth or subcutaneous injection three or four times a day, is of value in preventing bronchial spasm in asthma, but is less effective in relieving an attack already present. The action is slow in onset and is fully established only after about 1 hour, but lasts for about 4 hours. Sometimes ephedrine is injected subcutaneously with adrenaline to enhance and prolong the bronchodilator action of the latter. Atropine or belladonna may be given with ephedrine to relieve spasms and vomiting in whooping-cough and bronchitis in infants.
For allergic disorders, such as hay fever, urticaria, or serum sickness, ephedrine may be given alone or in conjunction with antihistamines, either by mouth or as a spray; eye-drops may relieve the conjunctival congestion of hay fever. The vasoconstrictive action of ephedrine on mucous membranes reduces congestion; it is less irritant than adrenaline and the effect lasts longer. Nasal drops or, preferably, sprays containing 0.5 to 2% of ephedrine or its salts are used to relieve allergic and vasomotor rhinitis and sinusitis, but their continued use is liable to aggravate the condition and cause rhinitis medicamentosa. For preparing solutions in oil, anhydrous ephedrine is preferred but the use of oily sprays and nasal drops is to be deprecated since it reduces ciliary activity and may cause lipoid pneumonia. Aqueous sprays should preferably be at pH 5-5 to 6-5.
Ephedrine is used as a mydriatic; solutions containing 2 to 5% dilate the pupil fully within 15 to 30 minutes but do not cause cycloplegia. The effect lasts 4 to 12 hours. Ephedrine is often ineffective in the presence of iritis or iridocyclitis, and also in highly pigmented eyes.
COLIC. Ephedrine hydrochloride is preferable to Morphia in both renal and biliary colic. In renal colic the results of � to 1 grain dose of ephedrine by mouth are spectacular � relief is almost instantaneous; in gallstone colic there is some relief but not to the same extent. A.J.Ambrose Med Pr. 1936. 196, 46.
ENURESIS. Ephedrine was given in 38 cases, over periods up to several months; the enuresis ceased in 10 cases and there was improvement in 14 others. After excluding urinary infection, � grain of ephedrine alkaloid in tablet form is prescribed to be given at bedtime the dose is increased by �grain every 3 to 4 nights until in some cases as much as 5 grains is being taken. The insomnia due to ephedrine may be relieved to some extent by giving �to 1 grain of phenobarbitone simultaneously. � R. W. Brookfield, Lancet, ii/1937, 623. Improvement occurred in 40 to 60% of 90 mentally defective patients, with ages ranging from 2 to 27 years, suffering from nocturnal enuresis, from the use of ephedrine hydrochloride in an optimal dosage of 1 grain at night. Higher-grade defectives reacted more favourably than lower-grade, and older patients than younger ones. � G. de M. Rudolf, per Abstr. World Med., 1948, 5, 238.
FIBROSITIS. A cream containing ephedrine 1, emulsifying wax 20, wool fat 10, chlorocresol 0-05, water to 100, was applied with deep massage. Patients were reported to remain free from pain while using the preparation once or twice daily. Most early cases were said to respond well; in most chronic cases there was temporary alleviation.�T. H. Howell, Med. Pr., 1950, 223, 514, per Pharm. J., ii/1950, 27. NARCOLEPSY. Symptoms successfully treated in 8 cases by ephedrine hydro-chloride or sulphate � grain 2 or 3 times daily.�H. Cohen, Lancet, ii/1932, 335. See also A. Haddow, ibid., 420.
In two cases amphetamine sulphate was given, but was of little value, but substitution of ephedrine sulphate ⅓ grain twice daily in one case, and thrice daily in the other led to some improvement.�G. Goswell, Med. J. Aust., i/1947, 272. Details are presented of a 4-year war record of a narcolepsy sufferer kept fighting fit on 10 half-grain tablets daily in 3 divided doses.� C. Swanton et al., Med. J. Aust., i/1955, 314.
NASAL DECONGESTION. A warning concerning the misuse of vasoconstrictor drugs (adrenaline and ephedrine) as sprays, oils or drops for nasal troubles. Continuous and indiscriminate use may be fraught with disastrous results. On repeated use the duration of the constriction becomes gradually reduced, and in the end a condition of aggravated dilatation is produced.�A. Francis, Brit. med. J., i/1936, 609.
An attempt to produce antiseptic or astringent effects in the nose with any of the solutions now in use is fraught with danger, and weak solutions of ephedrine ( �% in normal saline) are the only ones which should justifiably be used jto produce vaso-constriction, since these neither damage ciliary action nor interfere with the flow of mucus.�Brit. med. J., i/1939, 343.
OTITIS MEDIA. Ephedrine was used successfully as a decongestion agent in early cases of acute otitis media. A 2% aqueous solution of the alkaloid or sulphate was used, and pure glycerin when osmosis was also required. The majority of cases abort with this treatment if it is instituted early enough, and no risk appears to be involved. � W. O. Reid, Brit. med. /., i/1946, 648.
STOKES-ADAMS SYNDROME. Ephedrine sulphate � grain by mouth gave instant relief from attacks and in one week the individual dose was cut down to ⅓ grain. Medication ceased after a fortnight and there were no attacks for a year, when they were again relieved by ephedrine.�C. S. Higley and R. M. Stechen, per Brit. med. J., Epit., i/1934, 27. In 6 cases of complete heart-block, ephedrine orally increased the rate of ventricular beating in 4, and in 2 other cases complicated by Stokes-Adams seizures, ephedrine taken for 2� and l� years respectively was entirely successful in preventing syncopal attacks, but seizures returned with its discontinuance. The dose recommended is the minimum quantity consistent with an acceleration; � grain at 8-hour intervals may be sufficient.� A. R. Gilchrist, Brit. med. J., i/1934, 613.
Compound Ephedrine Spray (U.S.N.F.). Compound Ephedrine Inhalant. Ephedrine (dried over sulphuric acid) 1 g., camphor 600 mg., menthol 600 mg., thyme oil 0'3 ml., anhydrous light liquid paraffin to 100 ml.
STABILITY. This preparation is stable when kept in tightly closed containers, protected from light and stored in a cool place, provided that the light liquid paraffin used as solvent does not contain aldehydes or peroxides.� M. Pernarovski and L. G. Chatten, /. Amer. pharm. Ass., Sci. Edn, 1955, 44, 526.
Ephedrine Spray (U.S.N.F.). Ephedrine (dried over sulphuric acid) 1 g., methyl salicylate 0-2 ml., anhydrous light liquid paraffin to 100 ml.
For the preparation of Ephedrine Spray (U.S.N.F.) ephedrine containing less than 1% of water is required. Anhydrous ephedrine containing not less than 99% of C10H5ON, dried by storing over sulphuric acid is satisfactory. Ephedrine should not be dried in vacua over sulphuric acid as the base is too volatile at low pressures. Compound Ephedrine Spray (U.S.N.F.) may be prepared with ephedrine containing a little more moisture because the thyme oil, menthol and camphor have a solubilising effect.� S. W. Goldstein and D. P. Sanders, Drug Standards, 1955, 23, 34.
PROPRIETARY PREPARATIONS CONTAINING EPHEDRINE
Gluco-Fedrin (Parke, Davis). A solution containing ephedrine 1%, chlorbutol 0-5%, benzethonium chloride 0-02%, with menthol and dextrose in water. For the treatment of rhinitis.
Sedrin Plain (Lilly). An aromatic isotonic solution containing ephedrine alkaloid (combined with gluconic acid) 1%, and chlorbutol 0'5%. I-Sedrin Compound. An aromatic isotonic solution containing ephedrine alkaloid (combined with gluconic acid) 1%, and thiomersal 0-02%. For use as a spray or as nasal drops
.Noseptyl (Bengue). Liquid and jelly containing ephedrine 0-25%, menthol, and pine essence. For nasal congestion.
Anhydrous Ephedrine (B.P.C. 1949). Ephed. Anhydros.; Ephedrine. The anhydrous alkaloid prepared by vacuum distillation of the hydrate.
Foreign Pharmacopoeias: In Span., Fr., Mex., and U.S.N.F. specify anhydrous or hemihydrate). An unctuous, almost colourless, very deliquescent solid which tends to crystallise in needles and rapidly absorbs carbon dioxide. M.p. 33� to 37-5�. Solutions in water are alkaline to litmus. Solutions in oil are free from turbidity.Soluble 1 in 20 of water; readily soluble in alcohol; soluble in ether, chloroform, glycerin, olive oil, and liquid paraffin. Protect from light and moisture in a cool place.
The water content of ephedrine has a profound effect on the degree of solubility. At 20� and 25� anhydrous ephedrine is about two and a half times as soluble in liquid paraffin as the hemihydrate. At 25� anhydrous ephedrine is soluble in liquid paraffin to the extent of 2-87 g. in 100 ml. of solution, and in light liquid paraffin to the extent of 3'13 g. per 100 ml. of solution. The difference in the solubility of anhydrous ephedrine at 25� and 20� is about 20%.� J. Rosin et al., J. Amer. pharm. Ass., Sci. Edn, 1941, 30, 275. Anhydrous ephedrine is used for preparing oily solutions of ephedrine.
Ephedrine Hydrochloride (B.P., I.P.). Ephed. Hydrochlor.;
Ephe-drinium Chloride; Ephedrinum Chloratum; Ephedrinum Hydrochloricum (Hung;.
P.). C10H16ON,HCl = 201-7.
Foreign Pharmacopeias: In all pharmacopoeias examined except Ger. and U.S. but in Ger(ES). and U.S.N.F.
Colourless, odourless, Laevorotatory crystals with a bitter taste.
Soluble 1 in 4 of water, 1 in 14 of alcohol, and 1 in 60 of glycerin; insoluble in chloroform, ether, olive oil, and liquid paraffin. Solutions are sterilised by autoclaving or by filtration. Protect from light and moisture.
Uses. Ephedrine hydrochloride has the same action and uses as ephedrine, and in fact is the salt of ephedrine which is generally administered, either in aqueous solution by subcutaneous or intramuscular injection, or in tablets or elixir by mouth.
Capsules
Cream
Elixir
Dose: 2 to 8ml. (30 to 120 minims).
Nasal Drops
Nasal Drops of Ephedrine (B.P.C.). Narist. Ephed. (B.N.F.); Aqueous Spray of Ephedrine; Neb. Ephed.; Neb. Ephed. Aquos. Ephedrine hydrochloride 4 gr., sodium chloride 2 gr., chlorbutol 2 gr., water to 1 fl. oz.
Neb. Ephedrin. Aquos. Mit. (St. Thomas's Hosp.'). Ephedrine hydrochloride 2 gr., sodium chloride 2 gr., chlorbutol 2 gr., water to 1 fl. oz.
Tablets
Tab. Ephed. pro Inf. (B.N.F.). Ephedrine Hydrochloride Tablets for Infants. Unless otherwise specified, tablets each containing ⅛ grain of ephedrine hydro-chloride are supplied.
Tablets of Ephedrine Hydrochloride (B.P.). Tab. Ephed. (B.N.F.) Compressi Ephedrini Hydrochloridi. Unless otherwise specified, �-grain tablets are supplied.
If you did not find what you were seeking use the site search box at the top right hand of the page, or else peruse the site library.
![]()