
 Natural
Colouring Agents
Natural
Colouring Agents
Martindale�s 24th
Compiled and edited by Ivor Hughes.
Alkanna (B.P.C. 1949). Anchusa; Alkanet Root; Dyer's Alkanet. The
dried root of Alkanna tinctoria (Boraginaceae). It contains a red dye,
alkannin, C16H16O6, which is soluble in
oils and fats.
Uses. It is used for colouring toilet
preparations of an oily or spirituous nature, and as an alcoholic solution
in microscopy for the detection of oils and fats. 'Red oil' is prepared by
macerating alkanna 1 in liquid paraffin 7.
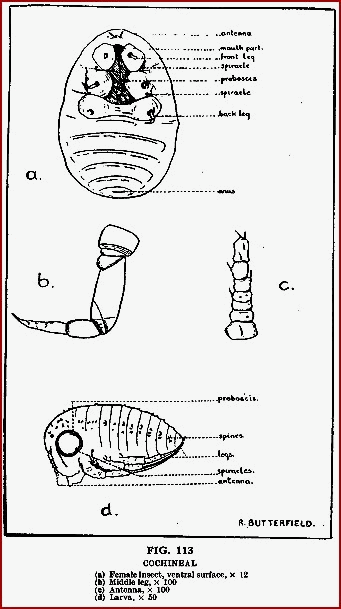
Cochineal (B.P.). Coccus; Coccinilla; Coccionella; Coccus Cacti.
Foreign Pharmacopoeias: In Dan., Egyp., Ind., and Swiss. Also in
U.S.N.F.
The dried female insect, Dactylopius coccus ( = Coccus cacti) (Coccidae),
containing eggs and larvae. If killed by sulphur or charcoal fumes the
insects are silver in colour ('silver grain') owing to a deposit of wax on
the surface. If killed by heat the wax melts and 'black grain' cochineal is
produced. Protect from light and moisture.
Uses. It is used, in the form of
tincture or solution, as a red colouring agent; the tincture was formerly
used as an indicator in volumetric analysis.
Liq. Cocc. (B.P.C. 1949). Cochineal Solution; Liquid Cochineal.
Digest cochineal 10 g. in a solution of potassium carbonate 1 g. in
water 60 ml. on a water-bath for 2 hours, replacing water lost by
evaporation; strain, cool, add alcohol (90%) 20 ml. and potassium citrate 10
g., and dilute to 100 ml. with water.
Liq. Ros. Dulc. (B.P.C. 1949). Sweet Solution of Rose.
Triturate cochineal 4 g. with potassium carbonate 4 g. and water 20 ml.,
add potash alum 4 g. and potassium acid tartrate 4 g., heat on a water-bath
for 1 hour and filter into glycerin 75 ml.; add rose oil 0.025-ml. in
alcohol (90%) 0.15 ml., and dilute with hot water to 100 ml.
Tinct. Cocc. (B.P. 1948, Ind. P.).
Cochineal 1 in 10 in alcohol (45%), prepared by maceration. Dan. P., 1
in 5.
Carmine (B.P.C.) The aluminium lake of the colouring matter of
cochineal.
Foreign Pharmacopoeias: In Belg., Egyp., Fr., and Swiss.
Light, bright red pieces, readily reducible to powder. Insoluble in water
and dilute acids; slightly soluble in alcohol; readily soluble in dilute
solution of ammonia and in dilute alkaline liquids, giving a dark
purplish-red solution. Protect from light.
Uses. It is used to colour medicinal and
toilet preparations, especially tooth powders and dusting-powders. To obtain
the maximum colour carmine should be dissolved in a small quantity of Strong
Solution of Ammonia and triturated with the powder. Carmine passes through
the stomach unchanged and is used as a 'marker' in metabolism
experiments and when it is desired to check the faeces corresponding to a
particular diet or method of treatment; it is given in a
dose of 200 to 500 mg. (3 to 8 grains) in a cachet or gelatin
capsule at the commencement of the treatment.
Liq. Carmin. (B.P.C. 1949). Solution of Carmine.
Triturate carmine 6 g. with dilute solution of ammonia 15 ml., add glycerin
35 ml., and heat on a water-bath for 5 minutes or until the carmine is
dissolved; cool, add potassium citrate 10 g., and dilute to 100 ml. with
water; filter if necessary. For colouring neutral or alkaline mouth-washes
or mixtures 3 or 4 m. per fl. oz. is used; the colouring matter is
precipitated in acid solutions.
Glycer. Carmin. (B.P.C. 1934). Glycerin of Carmine.
Digest carmine 6.25 g. in a solution of potassium carbonate 1 g. in
water 60 ml. on a water-bath until the carmine is dissolved; strain, cool,
add glycerin 20 ml. and potassium citrate 10 g., and dilute to 100 ml. with
water.
Saffron (B.P.C. 1949). Crocus; Stigma Croci; Safran; Estigmas de azafran;
Azafran.
Foreign Pharmacopeias: In Belg., Chil., Egyp., Fr., Ger., Ind., Jap.,
Jug., Mex., Nor., Pol., Span., and Swiss.
The dried stigmas and tops of the styles of Crocus sativus (Iridaceae).
Protect from light.
Uses. It has been used as a colouring
and flavouring agent in medicines.
Saffron has been employed as an abortifacient and several fatal cases have
been recorded. The chief toxic symptoms are flushing of the face, epistaxis,
vertigo, vomiting, bradycardia and stupor. Death (after profuse metrorrhagia
and abortion) has occurred following use of 24 grains.�P. Fasai and G.
Wachner, Wien. klin. Wschr., 1933, 45, 747
Glycer. Croci (B.P.C. 1934). Glycerin of Saffron.
Digest saffron 2.5 g. in alcohol (60%) 50 ml. and glycerin 50 ml. for 1
hour using gentle heat, and filter. Protect from air and light. As a
colouring agent 10 m. per fl. oz. is used.
Syr. Croci (B.P.C. 1934). Syrup of Saffron.
Glycerin of saffron 12-5 ml., syrup to 100 ml. Protect from light. As a
colouring agent 60 m. per fl. oz. is used.
Tinct. Croci (B.P.C. 1907). Tincture of Saffron.
Saffron 1 in 20 in alcohol (60%), prepared by maceration. As a colouring
agent 5 to 15 m. per fl. oz. is used. A 1 in 10 tincture is included in Belg.
P., Fr. P., Ind. P., Span. P., and Swiss P.
Cudbear (B.P.C. 1949). Persio.
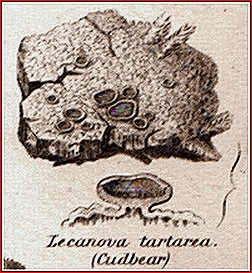 A
red colouring matter obtained from the lichens Roccella tinctoria, R.
montagnei and other species of Roccella (Roccellaceae). A purplish-red
powder which imparts a rich red colour to acid and neutral liquids, changing
to purplish-red on the addition of alkalis. Protect from moisture. Uses.
It has been used as a colouring agent especially in the preparation of
syrups having an acid reaction.
A
red colouring matter obtained from the lichens Roccella tinctoria, R.
montagnei and other species of Roccella (Roccellaceae). A purplish-red
powder which imparts a rich red colour to acid and neutral liquids, changing
to purplish-red on the addition of alkalis. Protect from moisture. Uses.
It has been used as a colouring agent especially in the preparation of
syrups having an acid reaction.
Tinct. Pers. (B.P.C. 1934). Tincture of Cudbear.
Mix cudbear 12-5 g. with washed sand 25 g., and prepare a tincture by
percolation "with a mixture of alcohol (90%) 35 ml. and water 70 ml.;
add more water if necessary to 100 ml.
Red Sanders Wood (B.P.C. 1949). Pterocarpus; Red Saunders (U.S.N.F.);
Pterocarpi Lignum; Lignum Santali Rubrum; Red Sandal Wood; Ruby Wood.
Foreign Pharmacopoeias: In Swed. Also in U.S.N.F. and Ind. P.C. The
heartwood of Pterocarpus santalinus (Leguminosae;). Uses.
It is used as a red colouring agent for alcohol preparations; the colouring
matter is precipitated when these are diluted with water.
Red-Poppy Petal (B.P.C. 1949). Rhoead. Pet.; Coquelicot; Klatschrose;
Petalos de amapola.
Foreign Pharmacopoeias: In Belg., Fr., Jug., Span., and Swiss.
The dried petals of Papaver rhoeas (Papaveraceae). Protect from light
and moisture in a cool place.
Uses. It is used in the form of Syrup of Red-Poppy as a colouring
and sweetening agent.
Syr. Rhoead. (B.P.C. 1949). Syrup of Red-Poppy.
To 40 ml. of hot water on a water-bath add gradually red-poppy petal 5.2
g., stirring frequently; remove the vessel from the water-bath and infuse
for 12 hours; strain, dissolve sucrose 72.5 g. in the infusion using gentle
heat, cool, add alcohol (90%) 5 ml., and dilute to 100 ml. with water.
Dose: 2 to 4 ml. (30 to 60 minims).
Chlorophyll.
This is a green colouring matter of plants which is soluble in organic
solvents and oils but only slightly soluble in water. It is a mixture of two
closely related substances, chlorophyll a and chlorophyll b.
Both constituents are magnesium complexes with a porphyrin structure
resembling that of haemoglobin and contain two hydroxyl groups, one of which
is esterified with phytyl alcohol and the other with methyl alcohol. The
only difference between the two chlorophylls is that a methyl side-chain in
chlorophyll a is replaced by a formyl group in chlorophyll b.
Oil-soluble Chlorophyll Derivatives. Treatment of the chlorophylls with dilute mineral acid causes the replacement of the magnesium atom by two hydrogen atoms with the formation of olive-green water-insoluble phaeophytins, which may be reconverted with difficulty to chlorophylls by treatment with methyl magnesium iodide. The magnesium can be replaced by other metals such as copper when copper phaeophytins (sometimes called copper chlorophyll) are formed; these are more stable to acids and to light than the chlorophylls.
Water-soluble Chlorophyll Derivatives. When the chlorophylls are hydrolysed with alkali, phytyl alcohol and methyl alcohol are split off and green water-soluble complex salts, still containing magnesium and called chlorophyllins, are formed. If sodium hydroxide is used for the hydrolysis, a mixture of sodium magnesium chlorophyllins a and b is the product. Treatment of these compounds with dilute acids results in the replacement of the sodium and magnesium atoms by hydrogen atoms to give water-insoluble derivatives, chlorin e from chlorophyll a and rhodin g from chlorophyll b, which on treatment with copper acetate and sodium hydroxide yield, respectively, water-soluble sodium copper chlorin e and sodium copper rhodin g (so-called sodium copper chlorophyllins). Similar water-soluble compounds can be prepared in which the magnesium is replaced by metals other than copper and which contain an alkali metal other than sodium.
Commercial Products. The pure chlorophylls and their derivatives described above are difficult to isolate and are not available commercially. In Great Britain, technical chlorophyll is obtained from lucerne, nettles, or grass, by extraction with alcohol or acetone; after removal of solvent and water-soluble extractives, the product consists of chlorophylls, carotenoids, phosphatides, fatty acids, etc., together with their decomposition products, the nature and amount of which depend on the extraction process.
Oil-soluble Products. Greenish semi-solid or fluid extracts containing up to 15% of chlorophyll or its derivatives are used chiefly for colouring soaps and oils. The chlorophylls are usually present as phaeophytins, a proportion of which may be copper phaeophytins arising from copper naturally present in the plant or from contact with copper or brass equipment. For colouring purposes the phaeophytins are usually converted completely into copper phaeophytin (to develop the maximum green colour and light- and acid-fastness) and diluted with a fatty oil such as palm or castor oil.
Providing excess of copper is not present the preparations are non-toxic and can be used in medicine. Whether copper-treated or not, the products are completely soluble in essential and fixed oils and fats and in hydrocarbon and chlorinated hydrocarbon solvents; they are partly soluble in methyl and ethyl alcohols (90%) and insoluble in dilute alcohols and water.
Water-soluble Products. Those prepared by alkaline hydrolysis of uncoppered oil-soluble phaeophytins are sometimes called 'magnesium chlorophyllins� although containing little or no magnesium and possibly a considerable proportion of copper; they are frequently called simply 'chlorophyllins'. When prepared from copper-treated oil-soluble products, in which the green colour is due to copper phaeophytin, the product is sodium or potassium copper chlorophyllin. Providing excess of copper is not present the products are non-toxic and can be used in medicine. Whether containing copper or not, the water-soluble products give alkaline solutions in water, from which they may be salted out by alkalis and mineral salts; acidification causes precipitation of insoluble acidic chlorophyllins leaving an almost colourless supernatant liquid. They are generally soluble in dilute alcohols (up to 60% v/v) although some grades are soluble in alcohol (90% v/v), but they are precipitated from alcoholic solutions by acids and alkalis; they are insoluble in organic solvents and in fixed and essential oils, although those grades that are soluble in alcohol (90%) are soluble in alcoholic solutions of essential oils.
Liquid or semi-solid products of varying strengths contain water and include a preservative such as glycerol to prevent fermentation and mould growth; they have a characteristic odour and an unpleasant taste. Purer grades, having only a faint odour and not unpleasant taste, are marketed as powders and as granules suitable for tableting.
The strong blue-green colour of the copper-containing products is fairly light-fast but fades rapidly in very dilute aqueous solutions; the dull green to brown colour of the copper-free products is more stable to heat and fades more slowly in dilute aqueous solutions. The properties, commercial grades, and deodorising and medicinal uses of chlorophyll derivatives are outlined.� W. Mitchell, Export Rev., 1952, 13, 29.
Uses.
Chlorophyll is employed principally as a colouring agent, especially for colouring fats, oils, soaps, etc. Chlorophyll is claimed to have a stimulant action on cell growth and has been used as an external application in the treatment of wounds and ulcers. There is no clear evidence that it does accelerate healing but it has a deodorant action on foul-smelling wounds when kept in constant contact in fairly high concentration and tends to give them a healthy granulating appearance. Commercial claims for the deodorant effect of chlorophyll tablets on halitosis and other body odours lack scientific proof, and the incorporation of chlorophyll in toothpastes for the same purpose is also of doubtful value.ANTIBACTERIAL ACTIVITY.
Alkali copper chlorophyllin at a dilution of 1 in 800 inhibited the growth of Staphylococcus aureus for 16 hours in vitro. After incubation for a further 24 hours, growth of Staph. aureus occurred in the presence of concentrations as high as 1 in 20 of the chlorophyllin, apparently owing to the development of resistance in the organism. The preparation did not inhibit the growth of Gram-positive organisms. S. Mowbray, Brit. med. J., i/1957, 268.DEODORANT ACTION.
Exposure of methanethiol gas in a closed system to water-soluble chlorophyll did not reduce the amount of gas passing over to an indicator, compared with a control. Exposure of small quantities of the gas to chlorophyll in a closed system for up to 3 days did not remove the smell of mercaptan. Mixtures of water-soluble chlorophyll and various strong-smelling solutions did not remove the smell of these solutions even after exposure of one or more months. Clinical trials are reported in which the small of urine, faeces, sweat, and of urine after ingestion of asparagus was not removed after the taking of large numbers of chlorophyll tablets.�J. C. Brocklehurst, Brit. med. J., i/1953, 541. Correspondence by manufacturers criticising the experiments and the findings.�ibid., 622. The evidence for a deodorising action is discussed and possible mechanisms for this action are suggested.�W. Mitchell, Mfg Chem., 1953, 24,152.The breath odour after drinking alcoholic beverages is due primarily to the presence of highly aromatic substances characteristic of each beverage. The reported deodorisation of beery breath with chlorophyllin suggests that this substance neutralises some of the characteristic aromatic substances present but experiment has shown that ingestion of 100 mg. of chlorophyllin is without effect on alcohol concentration in the blood and breath after consumption of whisky.�L. A. Greenberg and D. Lester, Quart. J. Stud. Alcohol, 1954, 15. 16, per J. Amer. med. Ass., 1954, 155,1115.
Chlorophyll solution was sprayed by atomiser 6 times a day for a week on and around the head, neck and dressings of 27 patients with odiferous carcinoma of the head and neck. Grossly stained tissues and dressings resulted without, unfortunately, total abolition of the disagreeable odour.�A. H. Kutscher et al., J. Amer. med. Ass., 1955,157,1279.
Chlorophyll Ointment.
Stearic acid 15 g., liquid paraffin 5 ml., macrogol '4000' 10 g.,
triethanolamine 1 g., sodium benzoate 1 g., chlorophyll (25% water-soluble)
4 g., water to 100 ml.�G. J. Sperandio, Bull. Amer. Soc. Hasp. Pharm.,
1951, 8, 292.
Melt together stearic acid 14 and cetyl alcohol 3. Dissolve triethanolamine 0.8, sodium lauryl sulphate 0.5 and glycerin 5 in sufficient water to make 100 of the final ointment. Mix the two liquids and incorporate 25% chlorophyllin solution 4 or sodium chlorophyllin 1.�H. Lehmann, Schweiz. Apoth. Ztg, 1953, 91, 187, per J. Amer. pharm. Ass., Pract, Pharm. Edn, 1955,16, 6.
Chlorophyll Suppositories.
Chlorophyll, oil-soluble, 6 g., theobroma oil to 60 g.�Pharm. J.,
ii/1951, 470.
PROPRIETARY PREPARATIONS CONTAINING CHLOROPHYLL.
Aniplex Clinical Tablets (Ashe Laboratories). Each contains 100 mg. of chlorophyll derivatives.
Chloresium (Rystan Ltd). Water-soluble derivatives of chlorophyll a, available as a 0.2% Solution in isotonic saline; and as a 0.5% Ointment in a hydrophilic basis. For topical application to wounds, ulcers, burns and dermatoses.
Duse (Scott &c Bowne). Tablets containing chlorophyll.
Flexaphyll (Fletcher, Fletcher & Co.).
Tablets each containing chlorophyll 50 mg.
Cream containing chlorophyll (sodium copper chlorophyllin) 0-5% and
phenoxyethanol 2% in a water-miscible basis.
Odoban (Brook, Parker). Tablets containing chlorophyll.
Did you find what you were looking for? If not please use the site search box, top right hand of the page. Or go to the Library.
![]()