
 CAMPHORA
� CAMPHOR. U.S. Br.
CAMPHORA
� CAMPHOR. U.S. Br.
2. United States Dispensatory 21st. 1926
3. Boericke�s Homeopathic Materia Medica.
Compiled and Edited by Ivor Hughes.
-::-
1.
The Extra Pharmacopoeia Martindale�s 24th British.Camphor (B.P., I.P.). Camph.; Camphre du Japon (natural); Camphre Droit (natural); Alcanfor. C10H16O = 152-2.
Dose: 120 to 300 mg. (2 to 5 grains); 60 to 200 mg. (1 to 3 grains) by injection. I.P. max. single dose 1 g., max. in 24 hours 5 g.
Foreign Pharmacopoeias: In all pharmacopeias examined.
It is obtained by distillation from the wood of Cinnamomum Camphora (Lauraceae) and purified by sublimation, or it may be prepared synthetically. The natural product is dextrorotatory and the synthetic product is optically inactive. Colourless transparent crystals, crystalline masses, blocks, or powdery masses known as 'flowers of camphor", with an aromatic odour and a pungent taste.
Soluble 1 in 700 of water, 1 in 1 of alcohol, 1 in 0-25 of chloroform, 1 in about 0'6 of ether, 1 in 1 -5 of oil of turpentine, 1 in 3 of olive oil and in other fixed vegetable oils; insoluble in glycerin; freely soluble in carbon disulphide and light petroleum. Oily solutions are sterilised by heating at 150� for 1 hour. Store in a cool place.
A liquid or soft mass is formed when camphor is triturated with many crystalline substances, e.g. beta-naphthol, chloral hydrate, phenol, salol, and thymol Camphor is powdered by triturating with a few drops of alcohol or other volatile organic solvent.
Toxic Effects
. Poisoning usually occurs from administration of camphorated oil to children in mistake for castor oil. The symptoms are nausea, vomiting, colic, dizziness, delirium, epileptiform convulsions and paralysis. Breathing is difficult, and the breath has a characteristic odour; anuria is common. Death is rare, and although fatalities in children have been recorded from 1 g., other children have survived as much as 5 g.Antidotes. Empty stomach by emetic or by gastric lavage with saline or milk. Give purgative dose of magnesium sulphate in plenty of water. Keep patient warm and give respiratory stimulants if necessary. Convulsions may be controlled with short-acting barbiturates. Asphyxia in a 20-month-old child following swallowing and aspiration of camphorated oil was successfully treated by atropine 1/300th grain and subcutaneous oxygen using a No. 20 short bevel needle in the flank for about 5 minutes.�N. M. Laurie, Canad. med. Ass. J., 1950, 63, 298.
Caution.
It is dangerous to place camphor or menthol, e.g. a 20% ointment, into the nostrils of an infant. A small quantity applied in this way may cause immediate collapse with signs of a severe syncopal attack.Uses. Taken internally camphor is irritant and carminative. As a camphorated linctus or syrup, or Camphorated Tincture of Opium it is employed as an expectorant in cough, and as Spirit of Camphor or Camphor Water it is used to relieve griping. Subcutaneously, a 10% solution of camphor in oil, is reputed to act as a respiratory and circulatory stimulant, but the effects are slight and inconstant. Applied externally, camphor acts as a rubefacient and mild analgesic and is employed in liniments for the relief of pain in neuralgia, myalgia, lumbago, and fibrositis. Camphorated chloral is used as a dental obtundent, and camphorated chalk is used as a dentifrice.
Elixir
Injections
In Belg. P. and Fr. P. (Huile Camphree Sterilisee Injectable) (10% w/v); in Egyp. P., Nor. P., and Swed. P. (20% w/v); in Chil. P. (20% w/w); Swiss P. includes both Injectabile Camphors Oleosum (10% w/w) and Injectabile Camphora Oleosum Fortius (20% w/w).
Inj. Camph. Aether. (B.P.C. 1934). Ethereal Injection of Camphor; Curschmann's Solution. Camphor 20% w/v and anaesthetic ether 30% v/v in olive oil; the ether is added aseptically to the sterilised camphor in oil.
Dose: 0-25 to 1 nil. (8 to 15 minims) subcutaneously.Injectabile Camphorae Aethereum (Swiss P.). Aether Camphoratus. Camphor 10%, w/v in anaesthetic ether.
Liniments
Camphor and Soap Liniment (U.S.N.F.). Soap Liniment. Camphor 45 g., hard soap 60 g., oil of rosemary 10 ml., alcohol 700 ml., water to 1000 ml.
Lin. pro Pernio (Adelaide Child. Hosp.). Chilblain Liniment. Camphor 10 g., menthol 10 g., cajuput oil 10 ml., strong solution of ammonia 10 ml., alcohol or industrial methylated spirit (90%) to 100 ml. For unbroken chilblains.
Liniment of Camphor (B.P., Ind. P.). Lin. Camph. (B.N.F.); Camphorated Oil. Camphor 20% w/w in arachis oil. In Egyp. P. and U.S.N.F. (20% w/w in cottonseed oil); in Cz. P. (20% w/w in sunflower-seed oil); in Dan. P., Jug. P., Nor. P., and Swed. P. (20% w/w in olive oil); in Belg. P., Fr. P., Mex. P. (10% w/w in olive oil). Ger. P. and Pol. P. include two strengths, 10 and 20% w/w in olive oil.
Linimentum Ammonia; Camphoratum (Dan. P.). Camphor 50 g., dilute solution of ammonia 250 g., rape oil 700 g.
Lotion
Ointments
Ung. Camph. Dur. (B.P.C. 1949). Hard Ointment of Camphor; Camphor Ice. Camphor 6 g., hard paraffin 26 g., white soft paraffin 68 g.
Spirits
Solute Alcoolique Faible de Camphre (Fr. P.). Camphor 2-5% w/w in alcohol (60%).
Solute Alcoolique Fort de Camphre (Fr. P.). Camphor 10% w/w in alcohol.
Spirit of Camphor (B.P.C., Ind. P., U.S.N.F.). Sp. Camph. Camphor 10% w/v in alcohol (90%).
Dose: 0-3 to 2 ml. (5 to 30 minims).Spiritus Camphors Fortior. Rubini's Solution. Equal parts by weight of camphor and dehydrated alcohol. For diarrhea
. Dose: 2 to 5 drops on sugar every 5, 10, or 15 minutes, according to the severity of the symptoms.Syrup
Waters
Camphor Water (B.P.). Aqua Camph.; Camphor Julep. Camphor 1 in 1000 of water. 60 minims of spirit of camphor added to 12 fi. oz. of water gives a solution of approximately the same strength. Dose: 15 to 30 ml. (� to 1 ft. oz.). U.S.N.F. specifies a saturated solution.
-::-
Monobromated Camphor (U.S.N.F.). Camphor Monobromide (B.P.C. 1934); Camphora Monobromata; Camphre Brome; Bromkampfer; Alcanfor bromuro. 3-Bromocamphor. C10H15OBr=231-l.
Dose: 120 to 300 mg. (2 to 5 grains); U.S.N.F. usual dose 125 mg.Foreign Pharmacopoeias: In Belg., Chil., Egyp., Fr., Span., and Swiss.
Colourless prisms with a persistent camphoraceous odour and taste. Almost insoluble in water; soluble 1 in 7 of alcohol, 1 in 2 of ether, 1 in 0-5 of chloroform and 1 in 8 of olive oil; sparingly soluble in glycerin; soluble in sulphuric acid with the formation of a nearly colourless solution. Incompatible with chloral hydrate, menthol, phenazone, phenol, salol, thymol, urethane, and alkalis. It is stable in air but is decomposed by prolonged exposure to sunlight. Protect from light.
Uses. It has been used in the treatment of headache and in certain chronic neurological conditions but is of doubtful value. Large doses may cause convulsions.
Sodium Camphorsulphonate (B.P.C. 1949). Sod. Camphorsulphon. Sodium (
+ )-camphorsulphonate. C10H15OSO2Na =
254-3.
Uses. Sodium Camphorsulphonate has the advantage over camphor in oil solution that it is more rapidly absorbed and has only very slight toxicity. It has been employed, for its stimulant action in the treatment of heart failure, syncope and shock, as a 15% aqueous solution given by parenteral injection. In cases of poisoning by carbon monoxide, gas, narcotics, etc., as much as 5 to 15 ml. may be given intravenously; in collapse during narcosis, 2 to 6 ml. may be injected intravenously, then up to 5 ml. intramuscularly.
Rectified Camphor Oil (B.P.C.). Ol. Camph. Rect.; Essential Oil of
Camphor; Light Oil of Camphor; White Oil of Camphor.
It consists of the lighter fractions of the oil obtained as a by-product in
the manufacture of natural camphor. It varies in composition according to
the extent to which camphor and safrole are removed. The heavier fractions
of the crude oil are used as a source of safrole and are known as 'brown oil
of camphor'. The rectified oil contains not less than 30% w/w of cineole. A
colourless or yellowish liquid with a characteristic camphoraceous odour and
taste. Soluble 1 in 3 of alcohol (90%). Wt per ml. 0-870 to 0-918 g.
Protect from light in a cool place.
Uses. Applied undiluted, or with an equal quantity of a vegetable
oil, it is used as a mild counter-irritant in the treatment of rheumatic
affections. It may also be used as a parasiticide.
2. United States Dispensatory 21st. 1926
CAMPHORA. U. S., Br. CAMPHOR Camph.
"A dextrorotatory ketone [C9H16CO] obtained from Cinnamomum Camphora. (Linne) Nees et Ebermaier (Fam. Lauracete)." U. S. " Camphor, C10H16O, is a white crystalline substance obtained from Cinnamomum Camphora, I. Nees and Eberm., purified by sublimation." Br.
Gum Camphor; Camphora Officinarum; Camphre du Japon Fr. Cod.; Camphre droit, Fr.; Camphora, P. G.; Kampher, Kampfer, Campher, (?.; Canfora, It.; Alcanfor, Sp.
The name of camphor has been applied to various concrete, white, odorous, volatile products, found in different aromatic plants, and resulting probably from chemical change in their volatile oil. But camphor proper is derived mainly from two plants, the Cinnamomum Camphora (L.) of Nees or Laurus Camphora of Linnaeus (Fam. Lauraceae) and the Dryobalanops Camphora Colebrooke (Fam. Dipterocarpaceae); the former of which yields our official camphor, the latter, the Borneo or Baras camphor, a product much valued in the East, but unknown in the commerce of this country and of Europe. Borneo camphor resembles true camphor, but is distinguished by being heavier than water and not being volatile at ordinary temperatures. On treatment with nitric acid it is converted into true camphor. In California, Bamona stachyoides, Artemisia tri-folium and Artemisia frigida are grown, for camphor.
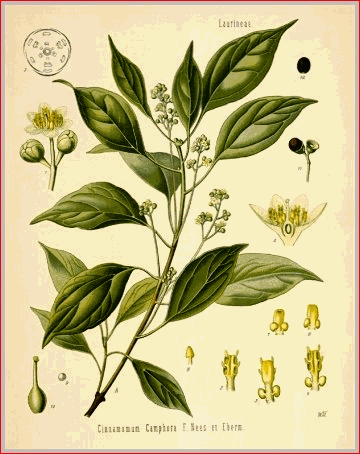
The camphor tree (Cinnamomum Camphora) is an evergreen which sometimes attains great size, having the aspect of the linden, with a trunk straight below, but divided above into many branches, which are covered with a smooth, greenish bark. Its leaves, which stand alternately upon long foot-stalks, are ovate-lanceolate, entire, smooth and shining, ribbed, of a bright yellowish-green color on their upper surface, paler on the under, and two or three inches in length. The flowers are small, white, pedicelled, and collected in clusters, which are supported by long axillary peduncles.. The fruit is a purple, one-seeded drupe, resembling that of the cinnamon. The camphor tree is a native of China, Japan, and adjacent portions of Eastern Asia, but is capable of cultivation in most sub-tropical countries, where the minimum winter temperature is not below -6.6� C., and the summers are warm. It thrives in Formosa, Madagascar, South Africa, Argentina, Egypt, Canary Islands, Southern Europe, and is being cultivated in India, Ceylon, the Federated Malay States, Luzon, Burma, Florida, Southern California, and Texas. The chief obstacle to its cultivation seems to be the extreme slowness of growth of the tree, and the corresponding slowness of returns from the investment. For an account of cultivation in Florida see D. C., Sept., 1902; also True and Hood, Proc. A. Ph. A., 1909, p. 719.
In Japan and Formosa the drug is obtained chiefly from the root, trunk and branches by the process of sublimation, but in the American plantations the leaves and small twigs are utilized, thus avoiding injury to the trees. Only the older trees are employed; indeed, it is said that a tree must be fifty years old before it should be considered available. In the province of Tosa, in Japan, the method of obtaining camphor, as reported by Dewey (Circular 12, U. S. Dept. of Agric., Div. of Botany), is as follows: " The trees are felled and the trunk, larger limbs, and sometimes the roots are cut into chips with a sharp, concave adze. The fresh chips are placed in a wooden tub about 40 inches high and 20 inches in diameter at the base, tapering towards the top like an old fashioned churn. The perforated bottom of the tub fits tightly over an iron pan of water on a furnace of masonry. The tub has a tight fitting cover, which may be removed to put in the chips. It is surrounded by a layer of earth about 6 inches thick to aid in retaining a uniform temperature. A bamboo tube extends from near the top of the tub into the condenser.
This consists of 2 wooden tubs of different sizes, the larger one right side up, kept about two-thirds full of water from a continuous stream which runs out of a hole in one side. The smaller one is inverted with its edge below the water, forming an air-tight chamber. This air chamber is kept cool by the water falling on the top and running down over the sides. The upper part of the air chamber is sometimes filled with clean rice straw, on which the camphor crystallizes, while the oil drips down and collects on the surface of the water. In some cases, the camphor and oil are allowed to collect together on the surface of the water, and are afterwards separated by filtration through rice straw or by pressure. About 12 hours are required for distilling a tubful by this method. Then the chips are removed and dried for use in the furnace, and a new charge is put in. At the same time the camphor and oil are removed from the condenser. By this method 20 to 40 pounds of chips are required for 1 pound of crude camphor."
In China the comminuted plant is said to be first boiled with water until the camphor adheres to the stick used in stirring, when the strained liquor is allowed to cool, and the camphor which concretes, being alternated with layers of earth, is submitted to sublimation. In the island of Formosa, where the camphor tree abounds, the chips are heated in a rough still. This is usually composed of a furnace surmounted with a trough or similar rude vessel, which is protected by clay. In this reservoir the chips are placed, with water upon them, and a perforated board luted upon the top; on this are set earthenware pots. A fire having been lighted, steam rises through the chips and carries the camphor with it to deposit it in the pots. The crude camphor is taken to the towns in baskets and then put into large vats, with holes in the bottom, through which an oil escapes called camphor oil, much used by the Chinese for medicinal purposes. It is said that of recent years hydraulic pressure is largely substituted for drainage.
Crude- camphor is of a pinkish color and of a softer consistency than the refined. It is purified by mixing it with about one-fiftieth of its weight of quicklime and heating in an iron vessel, first to a temperature of about 100� C. which drives off the water and volatile oil. Afterwards the vessel is connected with a suitable receiver and heated to a temperature of 175� to 200� C. which causes the camphor to sublime, the vapors condensing in the receiver. It is refined both in Japan and in the United States.
OIL OF CAMPHOR. � Two substances occur in commerce under the name of oil of camphor: The one derived from the Cinnamomum Camphora, known as the Formosa or Japanese oil of camphor, and formerly official under the name of Oleum Camphorae in the U. S. P.; the other the product of Dryobalanops aromatica, the East India oil of camphor, not occurring in American and European commerce.
Oil of camphor, as found in our markets, is a colorless fluid or of a light yellowish-brown color, having a strong odor like that of camphor, a bitterish camphorous taste and a specific gravity, according to Wm. Procter, of 0.940. It acts strongly on polarized light, and is dextrogyrate. Yoshida (A. J. P., 1886, p. 99) found in it besides two terpenes an oxygenated oil, which constituted half of the crude oil, to which he gave the formula C10H16O,H20, and calls it camphorogenol. Schimmel & Co., of Leipzig, have since extracted safrol commercially from the Japanese oil of camphor, and give a different account of its composition. They state (Schim. Hep., 1888) that it contains camphor, safrol, eugenol, a sesquiterpene, C15H24, and possibly terpinol. They do not consider Yoshida's camphorogenol to be a distinct substance.
Japanese oil of camphor varies in tint from the colorless transparency of water, through pale straw and yellow, to almost black. The specific gravity varies from 0.898 in the colorless oil to 0.990 in the very dark. The oil seems to vary greatly in the amount of camphor it contains, much of it having nearly all the solid principle removed before exportation. The odor is distinctly camphoraceous, with a peculiarity that suggests the odor of sassafras. It is said by Peter MacEwan to differ from the Formosa oil in its behavior towards nitric acid. If half a drachm of the acid be allowed to act upon half a drachm of Japanese oil, and then diluted with half a drachm of water, a crimson color will be produced.
The Formosa oil so treated yields a milky color with a scarcely perceptible green shade; hydrochloric acid gives with each oil a salmon color, more marked, however, with the Japanese oil. For further details, see P. J., vols. xv, xvi, and J. C. S., Oct., 1885. The oil is said to be used in Japan for the preparation of Chinese ink and varnishes, and for burning. As a diluent for artists' colors it is useful because its capacity for dissolving resins is greater than that of oil of "turpentine and similar liquids.
The oil of camphor has properties similar to those of camphor, but more stimulant, and is especially applicable to affections of the stomach and bowels in which an anodyne and stimulant impression is indicated, as flatulent colic and spasmodic cholera. It may also be used externally, as a rubefacient and anodyne liniment, diluted with soap, liniment, or olive oil, in local rheumatism and neuralgic pains, bruises, sprains, etc. The dose is two or three minims (0.12-0.2 cc.).
The Dryobalanops oil of camphor is said to be found in trees too young to produce camphor, and is supposed to constitute the first stage in the development of this substance, as it occupies the cavities in the trunk which are afterwards filled with the camphor. The chief constituent of it is a peculiar volatile oil, which is termed borneene; it is isomeric with oil of turpentine, C10H16, and holds in solution borneol and resin. By fractional distillation this oil may be separated into two portions, the one more volatile than the other, but not differing in composition.
Commercial History. � The commerce of the world is supplied with camphor chiefly from China, Japan, and Formosa, the great bulk of the product coming from the latter island. After the annexation of Formosa by the Japanese, that government, led by the unnecessary destruction of the camphor trees of the island, the inferiority of the camphor obtained, and the waste of the processes employed, and especially by the desire for revenue, made a governmental monopoly of the camphor trade which went into operation in 1900. The camphor is sold by the Japanese government through a single London firm from whose agencies refiners in different parts of the world buy it by contract for a year at a time. Camphor is now cultivated on a commercial scale in Florida. According to an article in the Oil, Paint, and Drug. Rep., Feb. 26, 1917, p. 18, one grove in Florida produces 40 tons of crude camphor annually.
Camphor is imported into the United States in tin-lined or zinc-lined cases. Crude camphor comes to the United States from Hong Kong and Foochow, China, and from Formosa; refined camphor from Shanghai, China and from Formosa; synthetic camphor from Germany.
Sumatra Camphor. Borneo Camphor. Dryobalanops Camphor. Bhimsaim Camphor. Baros Camphor, Borneol.�This camphor is produced in the islands of Sumatra and Borneo from Dryobalanops Camphora Coleb. This tree is very large, often exceeding one hundred feet in height, with a trunk six or seven feet in diameter and ranks among the tallest and largest trees in India. It is found in Sumatra and Borneo, and is abundant on the northwest coast of the former island. The camphor exists in concrete masses, which occupy longitudinal cavities or fissures in the heart of the tree, from a foot to a foot and a half long, at certain distances apart. The younger trees are generally less productive than the old. The only method of ascertaining whether a tree contains camphor is by incision.
When discovered, the tree is felled and cut into logs, which are then split, and the camphor removed by means of sharp-pointed instruments. It is stated that the masses are sometimes as thick as a man's arm, and that the product of a medium-sized tree is nearly eleven pounds; of a large one, double that quantity. The trees which have been wounded and left standing often produce camphor seven or eight years afterwards. Ida Pfeiffer states, in her Second Journey Round the World, that the camphor is also found in a concrete state under the bark and is swept down with long brooms. The whole tree is pervaded more or less by the camphor or the oil. The wood retains a fragrant odor, and, being on this account less liable to the attacks of insects, is highly esteemed for carpenter work.
Borneo camphor resembles in appearance ordinary camphor, but has, according to Christison, a specific gravity of 1.009. Its odor is also distinctly different from that of camphor. It usually pulverizes without the addition of alcohol, is less volatile than ordinary camphor and does not sublime on the interior of the bottles in which it is kept. It fuses at 206� C. and boils at 212� C.; is dextrogyrate; has a formula of C10H17(OH); and by the action of boiling nitric acid is converted into common camphor. It does not reach European commerce, being largely consumed in the Batta provinces, especially in funeral rites; and any that is exported is brought up at enormous prices for China, where it is preferred for embalming purposes on account of its being less volatile than the ordinary drug.
Borneo camphor is also produced in Johore, a province of the Malay peninsula, where it is sold in four qualities. The first is composed of transparent crystals, generally a quarter of an inch and upwards in length; the second of brown crystals, inferior in size; the third of powdery, coherent and slightly grayish colored crystals, which resemble Japanese camphor; the fourth quality is brownish, pulverulent, and looks like seashore sand.
Ngai camphor is yielded by the Blumea balsamifera DC. (Fam. Composite), a shrub from 5 to 6 feet high which occurs in India, China, Formosa, Philippines, etc. The crude drug is known to the Chinese as ngai-feu, and when refined in Canton as ngai-p'ien. About ten thousand pounds annually are exported from Canton. The refined camphor in appearance, odor, hardness, specific gravity, and volatility agrees almost precisely with Borneo camphor. According to Plowman, it has the chemical composition of Borneo camphor, but differs from it in its alcohol solution, being lawogyrate, and in being converted by boiling nitric acid into a substance thought to be identical with the stearopten of Chrysanthemum Parthenium Pers. Another species of Blumea, the B. grandis, a weed growing from 6 to 8 feet high, is said to yield a camphor identical with the Japanese product.
The physiological action of Borneo and Ngai camphor and of artificial borneol has been studied by E. Stockman (J. P., 1888, ix) who finds that the action of the three substances is practically identical and closely resembles that of true camphor. The results obtained by A. Lapin (Med. Age, 1894, xii) confirm those of Stockman, and show that in its proper dose the drug increases the energy of the heart, and that in frogs it is an especial motor nerve paralyzant.
Artificial Camphor .� Camphor can be made artificially by the oxidation of camphene, C10H16, with chromium trioxide mixture. Camphene is obtained from either pinene hydrochloride (so-called artificial camphor) or from bornyl chloride by treatment with alcoholic potash, and is a solid crystalline mass, fusing at 49� C. In the patented process of Nathan Thurlow, camphor is made by submitting oil of turpentine to the action of anhydrous oxalic acid in steam-jacketed reaction tanks. The liquid mass, containing pinyl oxalate and pinyl formate, is then distilled in the presence of an alkali with live steam, the resultant products being camphor, Borneol camphor, and certain oily products, which are separated from one another and purified by a somewhat complicated process. It is stated that turpentine yields in this process from 25 to 35 per cent, of its weight, and that oil of lemon and other essential oils, and terpenes are obtained as secondary products. This artificial camphor is either optically inactive or has a very slight dextrorotation, but in general physical properties exactly resembles natural camphor. So far, however, it seems to be difficult to work the process upon a large scale and the artificial product has had little effect upon the general camphor market.
Camphor can also be produced from isoborneol, by the action of oxidizing mixtures. The method patented in France, which is said to yield from 95 to 100 per cent, of camphor, consists in suspending ten kilogrammes of isoborneol in ten kilogrammes of benzol, and adding ten kilogrammes of potassium permanganate dissolved in one thousand liters of water, until the color of the permanganate is discharged. The camphor produced is separated by distillation and crystallized from petroleum benzin. Some pine needle oils and also Siberian oil of turpentine contain large proportions of bornyl acetate; the remaining terpenes may be converted into bornyl formate by the action of formic acid and a dehydrating agent. The mixed bornyl esters may then be converted into very pure camphor, free from chlorine compounds. A study of natural and synthetic camphor by Houseman giving complete review of all processes for producing the synthetic article and illustrated with structural formulas is found in A. J. P., 1915, p. 49.
While some writers have used synthetic camphor as a substitute for a natural variety with asserted good results, the facts that Pari and Bruni found the laevo-camphor thirteen times more toxic than the dextro-camphor and that the synthetic product is optically inert emphasizes the necessity of careful research before the artificial camphor can be considered therapeutically equivalent to the natural. (For literature see Borsich, Ph. Centralh., 1920, Ixi, p. 403.)
Camphor, C10H16O, and borneol, C10H18O, are classified together as belonging to the group called in general camphors, which are related to the terpenes, and are considered to be oxidation products of these. Borneol is an alcohol, yielding esters when heated to about 200� C. with organic acids. It is a secondary alcohol, and therefore contains the group CH.OH linked to a more complex group. Secondary alcohols by oxidation yield ketones, by the change of the CH.OH group to CO. Common camphor bears this relation to borneol, and is therefore considered as a ketone, and can be formed from borneol by the action of mild oxidizing agents. The action of metallic sodium, on the other hand, upon common camphor, C10H16O, yields borneol, C10H18O.
Description and Physical Properties. � " White, translucent, tough masses or granules. It has a penetrating, characteristic odor, and a pungent, aromatic taste. It is readily pulverizable in the presence of a little alcohol, ether, or chloroform. It slowly volatilizes at ordinary temperatures. One Gm. of Camphor is soluble in about 800 ec. of water, 1 cc. of alcohol, 0.5 cc. of chloroform, and in 1 cc. of ether, at 25� C. It is freely soluble in carbon disulphide, petroleum benzin, and in fixed or volatile oils.
"Specific gravity: about 0.990 at 25� C. Camphor melts between 174� and 177� C. The specific rotation [a]D of Camphor, determined at 25� C. in a solution containing 10 Gm. of Camphor in 100 cc. of alcohol and using a 200 mm. tube, is between + 41� and + 42�.
" A solution of Camphor in petroleum benzin (1 in 10) is clear (water). Gradually heat about 2 Gm. of Camphor: it sublimes without carbonization and without leaving more than 0.05 per cent, of non-volatile matter. Wind the end of a copper wire into a spiral about 6 mm. in diameter and 6 mm. in length, and hold it in a non-luminous flame until it glows without coloring the flame green. Dip the hot spiral in the Camphor, ignite, allow it to burn outside of the flame, and then bring the spiral into contact with the lower, outer edge of a non-luminous flame: not even a transient green color is imparted to the flame (chlorinated products).
" Preserve in well-closed containers, in a cool place." U. S.
" Colorless, transparent crystals or crystalline masses of tough consistence; also in rectangular tablets or in pulverulent masses known as ' flowers of camphor.' Penetrating and characteristic odor; taste pungent and somewhat bitter, followed by a sensation of cold.
Specific gravity about 0.995.
Melting point about 175� C.
Soluble in about 700 parts of water, in 1 part of alcohol (90 per cent.), in 0.25 part of chloroform, and in 4 parts of olive oil; very soluble in ether. Burns readily with a bright smoky flame, volatilises even at ordinary temperatures, and sublimes without residue when heated. Forms a liquid when triturated with chloral hydrate, menthol, phenol, thymol, and certain other substances. A solution of 5 grammes in sufficient alcohol (90 per cent.) to produce 20 millilitres exhibits at 15.5� C. an optical rotation of about +10� (distinction from synthetic camphor)." Br.
Camphor has a peculiar, strong, penetrating, fragrant odor, and a bitter, pungent taste, and a slight sense of coolness. It is beautifully white and pellucid, somewhat unctuous to the touch, brittle, and yet possessed of a tenacity which renders its reduction to a fine powder very difficult unless its cohesion be overcome by the addition of a minute proportion of alcohol, ether, chloroform, glycerin, essential or fatty oils, or other liquid for which it has an affinity. It may be obtained in powder by pulverizing with an equal weight of sugar, by precipitating the tincture with water, or by grating and afterwards sifting it, or by sublimation. A still better plan is to dissolve camphor in one and a half parts of alcohol, and pour this solution with stirring into four parts of water. Collect the precipitate, wash with water, and dry. By noting the quantity of camphor used, the amount left dissolved in the diluted alcohol can be calculated, and this solution used in making tincture.
The fracture of camphor is shining, and its texture crystalline. Its sp. gr. varies from 0.9857 to 0.996. When thrown in small fragments upon water, it assumes singular circulatory movements, which cease upon the addition of a drop of oil; and this property has been applied to the detection of grease in liquids, a very small proportion of which is sufficient to prevent the movements. Its volatility is so great that, even at ordinary temperatures, it is wholly dissipated if left exposed to the air. When it is confined in bottles, the vapor condenses on the inner surface, and, in large bottles partially filled, sometimes forms, after long standing, large and "beautiful crystals. It melts at 175� C., boils at 204� C., and, in closed vessels, sublimes unchanged. When allowed to concrete slowly from the state of vapor, it assumes the form of hexagonal plates. It is not altered by air and light. It readily takes fire, burning with a brilliant flame, with much smoke, and without residue.
Leo and Rimbach find that the solubility of camphor in pure water is 1 part in 598. The solubility in water falls with rise of temperature. (J. Soc. Chem. Ind., xxxviii, 1919, 738A.)
Carbon dioxide increases the solvent power of water, as also does the spirit of nitrous ether. Ordinary alcohol will take up 75 per cent, of its weight of camphor, which is precipitated upon the addition of water. Berzelius states that 100 parts of alcohol, of the sp. gr. 0.806, dissolve 120 parts of 10� C. It is soluble without change in ether, the volatile and fixed oils, strong acetic acid, and diluted mineral acids, and is extremely soluble in chloroform. Nitric acid on prolonged boiling with camphor oxidizes it into camphoric acid, C10H16O4, and camphoronic acid, C9H14O6. Schwanert's camphresinic acid, C10H14012, is, according to Kachler, a mixture of these two. Sulphuric acid in the proportion of ten parts to one gives, when heated with camphor, an oil isomeric with camphor, boiling at 200� C., and yielding a solid camphor when distilled repeatedly over potassium hydroxide. Sulphuric acid, in the proportion of four to one with camphor, gives, according to Chautard, a volatile product which he calls camphrene, and to which Schwanert gives the formula C9H14O. Kachler (Ann. Ch. Ph., clxiv, p. 90) considers, however, that camphrene is only phorone (a condensation product of acetone) with slight impurities.
Alcoholic potassium hydroxide solution heated with camphor gives a derivative called campholic acid, C10H18O2, a white solid, fusing at 95� C., and boiling at 250� C. Resins unite with camphor, forming a soft, tenacious mass, in which the odor of the camphor is sometimes almost extinguished, and frequently diminished; and a similar softening effect results when it is triturated with the solid fats. Exposed to a strong heat, in closed vessels, camphor is resolved into carbonic acid gas and hydrocarbons, among which cymol is especially to be recognized.
Genuine camphor is said to be sometimes adulterated with the synthetic, which may be detected by the action of ammonia upon its alcoholic solution, causing a flocculent precipitate, which does not re-dissolve, and the quantity of which is proportionate to that of the synthetic product in any mixture of the two. (A. J. P., xxxiv, 189.) As a means of distinguishing camphor from the synthetic camphor, J. W. Bailey recommends that a drop of alcohol, holding in solution a little of the camphor to be tested, be allowed to evaporate on the slide of a microscope. The crystals then formed produce, with polarized light, beautiful colors, if of natural camphor, but not, if of the synthetic. (Neues Repertorium, xvi, 763, 1867.)
The best test for distinguishing natural and synthetic camphor is by mixing 0.1 Gm. of the suspected substance, on a watch glass with ten drops of a cooled mixture of equal parts of vanillin-hydrochloric acid and concentrated sulphuric acid. Natural camphor first turns green and after five hours' exposure, dark blue; synthetic camphor remains yellow. Another point of distinction is said to be the fact that the synthetic camphor does not liquefy when triturated with an equal weight of chloral hydrate, while the natural camphor does.
Uses. � Locally camphor is irritant, with probably a benumbing influence upon the peripheral sensory nerves, and somewhat antiseptic. It is absorbed through mucous membranes not very readily, but easily from subcutaneous tissue. It combines in the body with glyeuronic acid and is eliminated in this combination by the kidneys. The systemic action of camphor is not well understood. In frogs it is depressant to the spinal cord and produces no motor disturbance except gradually increasing paralysis. In the mammalia, including man, it causes convulsions which are apparently due to an effect upon the motor tract of the brain. It appears also to have some stimulant action upon the intellectual centers and antagonizes the somnifacient drugs.
On the other hand, in conditions of nervous excitement it will frequently produce a sensation of quietude. The effects of camphor upon the circulation have been the subject of an immense amount of scientific investigation but with very contradictory results. The majority of authorities have found that the drug increases the force of the cardiac contractions in cold-blooded animals, but in the studies upon warm-blooded animals the results have been so contradictory that it seems impossible to harmonize them. Most investigators have found that camphor does not produce any consistent change in the blood pressure until large doses are given, when it is likely to cause a fall, but on the other hand, Pilcher and Sollman (J. P. Ex. T., 1915, vi, p. 349) have found that in the great majority of cases it causes a definite rise in the blood pressure. While Gottlieb (Z. E. P. T., 1905, ii) and others have obtained evidence of a stimulant action upon the heart muscle of warm-blooded animals, the majority of experimenters have been unable to determine such an effect. (For review of the literature on this subject, see J. P. Ex. T., 1914, v, p. 571.)
On the other hand, several investigators present evidence indicating that it has the power of antagonizing certain poisons such as strophanthin, chloral, phosphorus, etc., and the clinical evidence of the stimulating effect of camphor upon the circulation seems incontestable. Marfori, (Eif. M., 1918, xxxiv, p. 567) asserts that it is a remedy of great value in myocarditis with irregular rhythm; its action, he believes, is due to a stimulation of the nodal system of the heart. Marvin and Soifer (/. A. M. A., 1925, Ixxxiii, p. 94) in careful observations on fourteen patients with chronic heart disease, were unable to note any favorable influence of camphor upon either the circulation or general condition of the patient. For reference to the literature on this subject see the paper of Marvin and Soifer. In cases of heart failure whether due to diseases of the heart itself or secondary to infectious fevers, such as typhoid or in pneumonia, it is, however, highly esteemed by many clinicians.
In pneumonia camphor has been used not only as a cardiac stimulant but with the idea that it inhibits the growth of the pneumococci. While the evidence for this view is strongly suggestive it cannot be considered in any way conclusive. (For review of the literature on this subject see J. A. M. A., 1920, Ixxiv, p. 46.)
Aside from its action as a circulatory stimulant camphor is used internally for its calmative influence in hysteria, general nervousness and neuralgia. Whether the effects in these conditions are due to any action other than a psychic influence from the strong taste of the drug, is open to question. It is also employed in the treatment of serous diarrheas, its effects being probably due to a local irritant action upon the mucous membrane of the intestines. Externally it is widely employed as a counter-irritant in sprains, rheumatic affections, bronchitis, and other inflammatory conditions. It is also employed as a local application, especially in conjunction with menthol or phenol, in inflammations of the upper respiratory tract.
When a profound influence is desired, as in heart failure, it is necessary to give the drug hypodermically. For this purpose from three to five grains may be dissolved in twenty to thirty minims of sterile olive oil. The effect of a single injection lasts usually about two hours. Attention may be called to the fact that solutions of camphor in liquid petrolatum are entirely unsuited for hypodermic administration. Apparently the camphor is not absorbed under these conditions and not only does it fail to produce the desired physiological action but is often followed by serious local inflammatory changes. In nervous diseases it may be given in substance, in capsule, or as the spirit; the water contains so little camphor that it is scarcely more than a vehicle.
Cases of camphor poisoning, chiefly from the accidental ingestion of camphor liniment, have been reported. Benz (J. A. M. A., 1919, Ixxii, p. 1217) reports 20 such cases. The most constant symptom observed has been clonic convulsion, usually accompanied with vertigo, more or less mental confusion often amounting to active delirium and sometimes followed with coma. While sometimes the symptoms are very alarming the mortality is very low. The treatment, aside from evacuation of the stomach, is purely symptomatic.
Dose,
two to five grains (0.13-0.32 Gm.).Off. Prep. � Aqua Camphors, U. S., Br.; Ampullae Camphora, N. P.;
Ceratum Camphorae, N. F.; Ceratum Plumbi Subacetatis, N. P.; Linimentum
Aconiti, Br.; Linimentum Belladonnas, N. F., Br.; Linimentum Camphora, U.
S., Br.; Linimentum Camphorae Ammoniatum, Br.; Linimentum Chloroform! (from
Soap Liniment), (Br.); Linimentum Hydrargyri (from Camphor Liniment), Br.;
Linimentum Opii, Br.; Linimentum Saponis, U. S., Br.; Linimentum Sinapis,
Br.; Linimentum Terebinthinea Br.; Linimentum Terebinthineea Aceticum (from
Camphor Liniment), Br.; Spiritus Camphorae,, Br.; Tinctura Camphoras
Composita, Br.; Tinctura Opii Camphorata, U. S. (Br.); Unguentum 'Hydrargyri
Compositum, Br.; .Chloral Camphoratum, N. F.; Emplastrum Fuscum Camphoratum,
N. F.; Linimentum Ammonii lodidi, N. F.; Linimentum Opii Compositum, N. F.;
Linimentum Saponato-Camphoratum, N. W.; Linimentum Sinapis Compositum, N.
F.; Lotio Ammoniaealis Camphorata (from Spirit of Camphor), N. F.; Menthol
Camphoratum, N. F.; Mistura Camphorae Acida (from Water), N. F.; Mistura
Camphorae Aromatica (from Water), N. F.; Mistura Opii et Chloroformi
Composita (from Spirit), N. F.; Mistura Opii et Ehei Composita (from
Spirit), N. F.; Nebula Aro-maticum, N. F.; Nebula Mentholis Composita, N.
F.; Petroxolinum Chloroformi Camphoratum, N. F.; Petroxolinum Phenolis
Camphoratum, N. F.; Pilulas Antiperiodieaa, N. F.; Pilula Antiperiodicse
sine Aloes, N. F.; Pilulse Opii et Camphoraa, N. F.; Tinetura Antiperiodica,
N. F.; Tinctura Antiperiodicae sine Aloes, N. F.; Tinctura Pectoralis (from
Spirit), N. F.; Unguentum Camphorae, N. F.
3. Boericke�s Homeopathic Materia Medica.
Hahnemann says: "The action of this substance is very puzzling and difficult of investigation, even in the healthy organism because its primary action, more frequently than with any other remedy, alternates and becomes intermixed with the vital reactions (after effects) of the organism. On this account it is often difficult to determine what belongs to the vital reactions of the body and what to the alternating effects due to the primary action of the camphor."
Pictures a state of collapse. Icy coldness of the whole body; sudden sinking of strength; pulse small and weak. After operations, if temperature is subnormal, low blood pressure, 3 doses camph. 1x, 15-minute intervals. This condition is met with in cholera, and here it is that Camphor has achieved classical fame. First stages of a cold, with chilliness and sneezing. Subsultus and extreme restlessness. Cracking of joints. Epileptiform convulsions. Camphor has a direct relationship to muscles and fascia. In local rheumatic affections in cold climates necessary. Distention of veins. As a heart stimulant for emergency use of Camphor is the most satisfactory remedy. Drop doses on sugar as often as every five minutes.
It is characteristic of Camphor that the patient will not be covered, notwithstanding the icy coldness of the body. One of the main remedies in shock. Pain better while thinking of it. Very sensitive to cold and to touch. Sequelae of measles. Violent convulsion, with wandering and hysterical excitement. Tetanic spasms. Scrofulous children and irritable, weakly blondes especially affected.
Head.� Vertigo, tendency to unconsciousness, feeling as if he would
die. Influenza; headache, with catarrhal symptoms, sneezing, etc. Beating
pain in cerebellum. Cold sweat. Nose cold and pinched. Tongue cold, flabby,
trembling. Fleeting stitches in temporal region and orbits. Head sore.
Occipital throbbing, synchronous with the pulse.
Eyes. � Fixed, staring; pupils dilated. Sensation as if all objects
were too bright and glittering.
Nose � Stopped; sneezing. Fluent coryza on sudden change of
weather. Cold and pinched. Persistent epistaxis, especially with goose-flesh
state of skin.
Face .� Pale, haggard, anxious, distorted; bluish, cold. Cold
sweat.
Stomach. � Pressive pain in pit of stomach. Coldness, followed by
burning.
Stool. � Blackish; involuntary. Asiatic cholera, with cramps in
calves, coldness of body, anguish, great weakness, collapse, tongue and
mouth cold.
Urine. � Burning and strangury, with tenesmus of the neck of the
bladder. Retention with full bladder.
Male. � Desire increased. Chordee. Priapism. Nightly emissions.
Respiratory. � Praecordial distress. Suffocative dyspnoea. Asthma.
Violent, dry, hacking cough. Palpitation. Breath cold. Suspended
respiration.
Sleep. � Insomnia, with cold limbs. Subsultus and extreme
restlessness.
Extremities. � Rheumatic pain between shoulders. Difficult motion.
Numbness, tingling and coldness. Cracking in joints. Cramps in calves. Icy
cold feet, ache as if sprained.
Fever. � Pulse small, weak, slow. Icy coldness of the whole body.
Cold perspiration. Congestive chill. Tongue cold, flabby, trembling.
Skin. � Cold, pale, blue, livid. Cannot bear to be covered.
ISecale.]
Modalities. � Worse, motion, night, contact, cold air. Better,
warmth.
Relationship. � Camphor antidotes or modifies the action of nearly
every vegetable medicine�tobacco, opium, worm medicines, etc. Laffa
acuiangula (whole body ice-cold, with a restlessness and anxiety; burning
thirst). Camphoric acid�(a prophylactic against catheter fever; cystitis
15 grains three times a day; also for prevention of night sweats).
Incompatible: Kali nit.
Complementary: Canth.
Antidotes: Opium; Nitr. sp. dulc.; Phos.
Compare: Carbo; Cuprum; Arsenic; Verat*
CAMPHORA MONO-BROMATA (Mono-bromide of Camphor)
Nervous excitability is the guiding condition. Suppression of milk. Nightly
emissions. Painful erections. Paralysis agitans. Cholera infantum, and
infantile convulsions. Intensifies the action of Quinine and renders it more
permanent.
Mind.� Directions appear reversed, i.e., north seems south, and
east seems west. Hysteria; weeping and laughing alternately. Trance-like
state.
If you did not find what you were seeking then use the site search box at the top right hand of the page or peruse the site library .. if all else fails ask your questions in the site forum.
![]()