
 Cocaine Br.
USD 1926
Cocaine Br.
USD 1926
Compiled and Edited
by Ivor Hughes.
Introduction:
The God
Coca has good cause to sit and ponder the cupidity and stupidity of men.
He is probably wondering how his beneficent gift became a scourge whose
lash sprouts corruption at every lick.
What the British did with Opium in China the Americans are currently doing in North and South America with Cocaine. The past and present history of this chicanery is well and honestly documented elsewhere. Suffice to say that when the powerful meddle for money, it is the poor that feel the pain.
Those who practice the healing arts understand how this plant either in its crude form or prepared as an arbitrary tincture has been transformed into its current evil. I am talking of course of its alkaloids, its salts and the synthetics which is the practice of orthodoxy in the use of such potent agents.
1. Martindale�s 24th Edition. 2. U.S.D. 1926
1. Martindale�s 24th Edition.
Coca (B.P.C. 1934). Coca Leaves.
Dose: 1 to 4
g. (15 to 60 grains). Foreign Pharmacopoeias: In Belg., Chil.
Egyp. Fr., Span., and
Swiss.
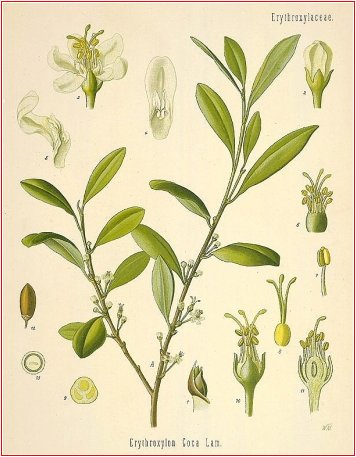
The dried leaves of Erythroxylum coca (Bolivian or Huanuco leaf) or of E. truxillense (Peruvian or Truxillo leaf) (Erythroxylaceae), indigenous to Bolivia and Peru and cultivated in Java.
The total alkaloid content varies from 0-5 to l-5%. Truxillo and Java leaf contains more, but only about half is cocaine. The total alkaloid of Bolivian leaf contains 70 to 80% of cocaine. Coca is now frequently valued on its ecgonine content since commercial cocaine is obtained synthetically from ecgonine.
Over 7 million kg. of coca leaf is consumed annually in Peru, mostly by the poorer classes. Daily individual consumption ranges from 20 to 100 g., divided into 10 to 20g. doses. The tolerance does not increase and addicts take the same dose for years. The most important immediate effects are suppression of hunger, thirst, cold, and tiredness. C. G. Noriega, per Abstr. World Med., 1948, 4, 246.
Bolivia is said to produce 3 million kg of coca leaves per year of which 85% is for Library consumption. Passmore, Lancet, i/1949, 362.
Uses. Coca was formerly employed, usually in the form of an elixir or liquid extract, as a cerebral and muscle stimulant during convalescence, and for the relief of gastric pain, nausea, and vomiting, but it is now seldom used in medicine. In Bolivia and Peru the leaf is chewed by the native workers to relieve hunger and fatigue.
Elix.
Cocas (B.P.C. 1934).
Elixir of Coca.
Liquid extract of coca 16-5 ml., simple
elixir to 100 ml. Dose: 4 to 16 ml. (60 to 240 minims).
Ext. Cocas Liq. (B.P.C. 1934).
Liquid Extract of Coca; Miscible Liquid Extract of
Coca.
Prepared by percolation with alcohol (60%) and adjusted to contain 0-5%
w/v of ether-soluble alkaloids, calculated as cocaine.
Dose:
2
to 4 ml. (30
to 60 minims).
2. U.S.D. 1926
COCAINA.
U. S., Br.
COCAINE Cocain.
An
alkaloid [C17H21O4N] obtained from the
leaves of Eryihroxylon Coca Lamarck and other species of Eryihroxylon. (Tarn.
Erythroxylaceae). "U. S."
Cocaine, C17H21NO4, is an alkaloid
obtained from the leaves of Erythroxylum Coca, Lam., and its varieties."
Br. Benzoyl-ecgonine-methyl-ester;
Benzol-methyl-ecgonine; Cocainum; Cocaine, Fr. Cod.; Cocain, Kokain, G.
Squibb's process for cocaine and its hydrochloride is as follows: Coarsely ground coca leaves are repercolated with an aqueous five per cent, solution of sulphuric acid, and a very dense, slightly acid percolate is obtained; this is thoroughly agitated with pure coal oil and an excess of sodium carbonate; the liberated alkaloid is retained by the coal oil, and is nearly free from coloring matter; the oily solution is then agitated with acidulated water, and again precipitated by sodium carbonate in the presence of ether. The ethereal solution of cocaine is treated with diluted hydrochloric acid fractionally, and the nearly colorless solutions of cocaine hydrochloride are cautiously evaporated in shallow porcelain pans almost to dryness.
The product is in the form of a white, crystalline, granular powder, and is a nearly pure anhydrous salt. (Ephem.,1887, 906.) For Henrique's process see Proc. A. Ph. A., 1895, 999.
Owing to the small yield, it is found more profitable to manufacture cocaine in South America and export it, thus saving the expense of transporting the bulky coca leaves.
The
cocas contain a number of alkaloids which Henry (Plant Alkaloids, 1924,
p. 95) divides into four groups;
(1)
the cocaine�s,
(2) pseudo-tropeines,
(3)
acylecgonines,
(4) hygrines.
In the first group is cocaine (methyl benzoyl ecgonine), cinnamyl
eocaine (methyl cinnamoyl ecgonine), and alpha and beta truxilline, C38H46O8N2.
Alphatruxilline is also known as
cocamine and
isatropyl cocaine.
The second group comprises one alkaloid, tropacocaine, which is a benzoyl-pseudotropein. This alkaloid is important not merely because of its use in medicine but also because the base pseudotropein is isomeric with the base tropine found in atropine.
In the fourth group are hygrine, C8H15ON, cuscohygrine, C13H24ON2 and betahygrine, C14H24ON2. These are comparatively simple compounds and are liquid at ordinary temperatures.
With the exception of ecgonine and anhydro ecgonine, all of the bodies in the foregoing list are easily decomposable, splitting up when heated to from 80� to 100� C. with hydrochloric acid, or when boiled with alcoholic potassium hydroxide.
Cocaine has the following structural formula:
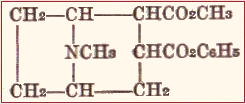
The characteristic group conferring the anesthetic properties is the benzoyl group. It may be identified by treatment with strong acids and heating, the decomposition into methyl alcohol and benzole acid being followed by the production of methyl benzoate with its very characteristic odor. It is easily decomposed by heat in the presence of moisture on solutions of its salts may not be sterilized by heat.
Much of the cocaine used in this country is prepared from the crude alkaloid which is manufactured in the countries where the plant grows. The alkaloids are extracted from the leaves by kerosene or some other cheap immiscible solvent in the presence of an alkali, the alkaloids being separated from the solvent with dilute sulphuric acid from which cocaine is precipitated by sodium carbonate. This crude substance represents about 90 per cent, of cocaine.
A good deal of cocaine is manufactured in Germany from the Java coca leaves which contain chiefly cinnamyl cocaine. This alkaloid is hydrolized by boiling with diluted hydrochloric acid and the ecgonine so obtained treated with benzoic anhydride and methyl iodide. The other coca bases may also be converted into cocaine by a similar process as suggested by Liebermann (B.Chem.G.,1888, xxi, 3196).
Description and Physical Properties. Colorless crystals, or a white, crystalline powder. It is odorless, and is stable in the air. One Gm. of Cocaine is soluble in about 600 cc. of water, 6.5 cc. of alcohol, 0.7 cc. of chloroform, 3.5 cc. of ether, 12 cc. of olive oil, and in 30 to 50 cc. of liquid petrolatum, at 25� C. One Gm. is soluble in 270 cc. of water at 80� C. It is very soluble in warm alcohol.
Cocaine melts between 96� and 98� C. Its saturated aqueous solution is alkaline to litmus paper. Its solution in diluted acids is laevorotatory. Heat about 0.1 Gm. of powdered Cocaine with 1 cc. of sulphuric acid for five minutes at 100� C., then cautiously mix with 2 cc. of distilled water: the aromatic odor of methyl benzoate is noticeable and on cooling crystals of benzoic acid separate.
The ash from 0.5 Gm. is negligible. Dissolve 0.1 Gm. of finely powdered Cocaine in 1 cc. of sulphuric acid: not more than a slightly yellow tint is produced (readily carbonizable substances). Dissolve 0.3 Gm. of finely powdered Cocaine in 1 cc. of normal hydrochloric acid, gently warming, if necessary, to aid solution, and dilute with distilled water to 15 cc.: 5 cc. portions of this solution do not respond to the tests for cinnamyl-eocaine and for isoa-tropyl-cocaine under Cocaines Hydrochloridum. U.S.
The British Pharmacopoeia gives the following description: In colorless monoclinic prisms. No odor; taste bitter, followed by a sensation of tingling and numbness. Soluble in 10 parts of alcohol (90 per cent.), in 4 parts of ether, in 0.5 part of chloroform, and in 24 parts of olive oil; almost insoluble in water} Melting point 98� C. The dry salt obtained by dissolving Cocaine in water acidified, with hydrochloric acid, and evaporating the solution, responds to the tests described under ' Cocainae Hydrochloridum. Br.
Uses.: For most
purposes cocaine hydrochloride is preferred to the base, but the latter
is used in ointments and oily solution because of its greater solubility
in fatty substances. Other salts of cocaine have been used in medicine.
Cocaine borate has been recommended for ophthalmological uses Cocaine
hydriodide which occurs in colorless crystals moderately soluble in
water, has been recommended by
R. Marcus as being especially suitable for use by cataphoresis for the
production of anesthesia. For medicinal uses, see Cocaine Hydrochloride.
Dose, one
eighth to one fourth grain (0.008� 0.016 Gm.).
Off. Prep.
Oleatum Cocainae, N. F.; Unguentum Cocainae, Br.